Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research - (2021)Volume 12, Issue 8
A Simple, specific, accurate, precise, rapid, robust and selective stability-indicating reverse-phase high-performance liquid chromatography (RP-HPLC) method has been developed, Validated for the simultaneous estimation for the antifungal (Luliconazole) and anti-inflammatory (Clobetasol Propionate) drugs. Forced degradation studies were done for the evaluation of the stability of the product. The mobile phase used in the method development and validation was Acetonitrile: Water: Methanol (70:15:15). The column used was Waters (Sunfire) X Bridge C18 column (250 mm×4.6 mm, 5 um) with a flow rate of 1 mL/min. Both the drugs were simultaneously detected at 250 nm and the Retention Time was found to be 1.87 min for Luliconazole and 4.35 min for Clobetasol Propionate. The method has been linear for both drugs. The Linearity of Luliconazole was found at 8-12 ug/mL and 0.40-0.60 ug/mL for Clobetasol Propionate. The Regression Coefficient was found to be 0.9997 for Luliconazole and for Clobetasol Propionate 0.9998. The method has been robust under various Conditions with variations in flow rate, Temperature and Wavelength. The developed method can be used routinely for simultaneous estimation of drugs Luliconazole and Clobetasol Propionate.
Luliconazole is chemically an imidazole derivative which prevents fungal infection [1-2] (Figure 1). It is useful in treating tinea corpis, tinea cruris and tineapedis. It acts on the 14 alpha demethylase of cytochrome p450 and inhibits the production of ergosterol which is a building block of cell membrane and leads to the destruction of fungi cell membrane [3,4]. 21-chloro-9α- fluoro-11β, 17α-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17- propionate is a chemical name of Clobetasol Propionate (Figure 1). It belongs to the class of corticosteroid [5]. It is a super-potent synthetic di-halogenated analogue of prednisolone. It acts by inhibiting the precursor of arachidonic acid (COX-2 and Phospholipase A) by binding to the receptor and sending signal to decrease proinflammatory proteins, thereby decreasing inflammation [6-7]. Therefore, Clobetasol is used topically on the skin to treat swelling, itching and irritation. It is used for the prevention of Psoriasis also (skin disease that causes red, itchy scaly patches, most commonly on the knees, elbows and scalps) [8-10].
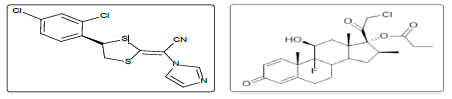
Figure 1: Chemical Structure of the drugs: (a) Clobetasol Propionate (b) Luliconazole
The mentioned drugs have been reported either individually or in a combination of two or separately but do not involve in simultaneous estimation of Luliconazole and Clobetasol Propionate. Literature survey revealed the method for determination of Luliconazole and Clobetasol Propionate with other drug combinations like RP-HPLC [11-13], UV Spectroscopy [14-17], High-Performance Thin Layer Chromatography [18- 20]. But no methods have been reported for simultaneous determination of Luliconazole and Clobetasol Propionate in the combined topical dosage form. Hence a successful attempt has been made to estimate these drugs simultaneously by RPHPLC method in the present work. The proposed methods were optimised and validated as per ICH guidelines.
Experimental chemicals
RP-HPLC method: The chromatographic analysis was performed on HPLC system of WATERS (Milford, USA) composed of 515 HPLC pump as a solvent delivery system equipped with Rheodyne injection valve with a 20 μL loop, WATERS 2498 detector and separation was performed on C18 column (4.6 mm × 150 mm, 5 μm i.d.) at 25°C column temperature. Chromatographic data were recorded and processed using EMPOWER-2 software.
Reagents and materials
Working standard grade of Luliconazole and Clobetasol Propionate was supplied by ISF Analytical Laboratory (Punjab, India), Luviv- CT, marketed formulation with label claim 1% of Luliconazole and 0.05% Clobetasol Propionate. Acetonitrile, Methanol, Water of HPLC grade, 0.45 mm PVDF filters were also supplied by Sd-fine Ltd.
Optimization of chromatographic conditions
Selection of proper HPLC method depends upon nature of drugs (ionic or neutral molecule), its molecular weight and its solubility. RP-HPLC method was selected initial separation because of its simplicity, efficiency, reproducibility, and recommended use for ionic and moderate to non-polar compounds. To optimize the chromatographic conditions, chromatographic variables such as mobile phase, pH, flow rate, column temperature, and solvent ratio were studied. The condition that gave best resolution, symmetry, theoretical plates and capacity factor was selected for estimation.
Optimized chromatographic condition
The optimised condition after many trials has been given in Table 1.
| Mobile Phase | ACN: Water: MeOH |
|---|---|
| Ratio | 70:15:15 (0.1 %Formic Acid) |
| pH | 3 |
| Column | C18 Sunfire |
| Wavelength | 250nm |
| Detector | UV Detector |
| Injection Volume | 20uL |
| Diluent | Methanol |
| Flow Rate | 1mL/min. |
| Run Time | 10min. |
Table 1: Optimized Chromatographic Conditions .
Preparation of mobile phase
The mobile phase was prepared by mixing the HPLC grade Acetonitrile, Methanol, Water at ratio of (70:15:15). Maintain the pH at 3 by Formic acid. Filter it by 0.45 um filter membrane, Sonicate for 25 minutes.
Preparation of standard solution and test solutions
Standard Solution for Luliconazole: Weigh 10 mg of Luliconazole was transferred in 10 mL of Volumetric Flask make up the volume up to methanol (1000 ug/mL) From the above stock pipette out 1 mL and transfer to 10 mL volumetric flask and make up the volume with Methanol (100 ug/mL). Pipette out 1 mL from the 100 ug/mL and transfer to 10 mL volumetric flask and make up the volume by Methanol.
Standard solution for clobetasol propionate: Weigh 10 mg of Clobetasol Propionate was transferred in 10 mL of volumetric flask make up the volume up to methanol (1000 ug/mL) From the above stock Solution pipette out 1 mL and transfer to 10 mL volumetric flask and make up the volume with methanol (100 ug/mL). Pipette out 1 mL from 100 ug/mL and transfer to 10 mL volumetric flask and make up the volume by methanol (10 ug/mL). Now pipette out 0.5 mL from 10 ug/mL and make the volume in 10 mL volumetric flask.
Combined standard solution: 1 mL of Luliconazole Dilution was pipette out and 4 mL of Clobetasol Propionate Dilution were pipette out and mix well, filter and from this mixture 20 uL was injected through rheodyne syringe.
Preparation of calibration curve: The calibration curve was prepared by injecting concentration of 0.40-0.60, 8-12 μg/mL of Luliconazole and Clobetasol Propionate and tertiary mixture solutions manually in triplicate to the HPLC system at detection wavelength of 250 nm. Mean of n=5 determinations was plotted as the standard curve. The calibration curve was tested and validated with interday and intraday measurements.
Method validation
The proposed method's analytical validation parameters were determined according to International Conference on Harmonization (ICH) guidelines. Analysis of sample was carried out using the above method and the result are show in Table 1.
Linearity and range
Linearity of the method was established by analysis of combined standard solution. The range of an analytical procedure is the interval between the upper and lower concentrations (amounts) of analyte in the sample (including these concentrations) for which it has been demonstrated that the analytical procedure has a suitable level of precision, accuracy and linearity. Mixed standard solution of Luliconazole and Clobetasol Propionate were prepared with methanol in such a way that the five different concentrations of Luliconazole and Clobetasol Propionate in the range of 8-12 μg/mL and 0.40-0.60 μg/mL respectively. The peak area was recorded for all the peaks as shown in Table 2 for linearity of Luliconazole and Clobetasol Propionate Tables 3 and 4. The plots of peak area versus the respective concentration were found to be linear with regression coefficient (r2=0.9997) for Luliconazole and (r2=0. 0.9998) for Clobetasol Propionate as shown in Figure 2.
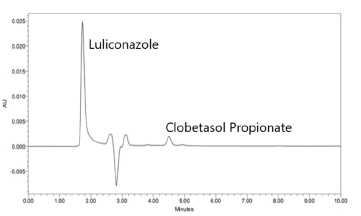
Figure 2: Chromatogram of Luliconazole and Clobetasol Propionate
| Drug | Label claim (mg) | Amount found (mg) | % Estimation (mg) |
|---|---|---|---|
| Luliconazole | 1000 mg | 992 mg | 99.28 |
| Clobetasol Propionate | 50 mg | 50.02 mg | 100.04 |
Table 2: Assay of Luliconazole and Clobetasol Propionate.
| Conc. (µg/mL) | Area |
|---|---|
| 8 | 253405 |
| 9 | 274311 |
| 10 | 293780 |
| 11 | 315989 |
| 12 | 336789 |
Table 3: Conc. vs. Area.
| Conc. (µg/mL) | Area |
|---|---|
| 0.4 | 14722 |
| 0.45 | 16699 |
| 0.5 | 18885 |
| 0.55 | 20985 |
| 0.6 | 23120 |
Table 4: Conc. vs. Area.
For accuracy study data from nine determinations over three concentrations at 80%, 100% and 120% of expected sample concentration covering the specified range was determined and expressed as recovery values. Accuracy of the proposed method was evaluated by spiking standard stock solution containing Clobetasol Propionate and Ketoconazole into placebo equivalent to amount present in sample preparation to achieve at 80%, 100% and 120% of the target concentration. Measured recovered concentration versus added concentration for Luliconazole and Clobetasol Propionate calculated percentage recovery. percentage recovery for all components were found more than 97.0%, this indicate accuracy of the method as shown in Figure 3. Recovery results are tabulated in Tables 5 and 6.
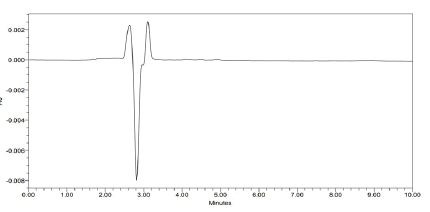
Figure 3: Blank chromatogram
| Recovery Sample | Fortified Sample | ||||||
|---|---|---|---|---|---|---|---|
| Sr.No. | Conc. (%) | Peak Area | Mean Area | Conc. (%) | Peak Area | Mean Peak Area | % Recovery |
| 1 | 80 | 253405 | 253419 | 80+100 | 610174 | 546907 | 99.98 |
| 254334 | 610176 | ||||||
| 2 | 100 | 293780 | 293782 | 100+100 | 966319 | 587564 | 100 |
| 293814 | 966300 | ||||||
| 3 | 120 | 336789 | 336784 | 120+100 | 1009593 | 630566 | 100.01 |
Table 5: Percentage Recovery for Luliconazole.
| Recovery Sample | Fortified Sample | ||||||
|---|---|---|---|---|---|---|---|
| Sr.No. | Conc. (%) | Peak Area | Mean Area | Conc. (%) | Peak Area | Mean Peak Area | % Recovery |
| 1 | 80 | 14722 | 14724 | 80+100 | 182345 | 31424 | 100.2 |
| 14727 | 182337 | ||||||
| 2 | 100 | 16699 | 16700 | 100+100 | 201346 | 33400 | 100.6 |
| 16702 | 201356 | ||||||
| 3 | 120 | 23120 | 23122 | 120+100 | 257896 | 39488 | 98 |
| 14722 | 182345 | ||||||
Table 6: Percentage Recovery for Clobetasol Propionate.
Precision
Intermediate Precision: The method is validated for intermediate precision by analyses of homogenous mixture of 100% test concentration of both drugs in six replicate preparations using two different analysts in two different days. For first day, same analyst analyzing the six preparations of samples and for second day different analyst utilizing same chromatographic conditions. The results obtained showed percentage RSD below 2% which is under accepted range as shown in Figure 4. Hence method is precise as described in Tables 7 and 8.
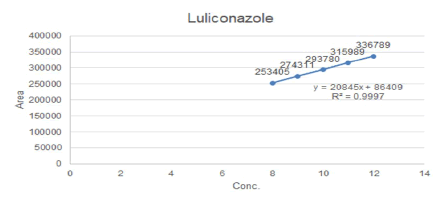
Figure 4: Linear graph of Luliconazole with R2=0.9997
| Conc. 10 µg/mL | Analyst -1 | Conc. 10 µg/mL | Analyst-2 |
|---|---|---|---|
| 1 | 293167 | 1 | 296868 |
| 2 | 293267 | 2 | 292874 |
| 3 | 293356 | 3 | 292923 |
| 4 | 293489 | 4 | 293104 |
| 5 | 293823 | 5 | 293237 |
| 6 | 293451 | 6 | 293567 |
| Mean | 293425.5 | Mean | 293762.2 |
| ± S. D. | 207.9517 | ± S. D. | 1407.499 |
| % R.S.D.(= 2) | 0.07087 | % R.S.D (= 2) | 0.479129 |
Table 7: Intermediate Precision of Luliconazole.
| Conc. 0.40µg/ml | Analyst -1 | Conc. 0.40 µg/ml | Analyst-2 |
|---|---|---|---|
| 1 | 14589 | 1 | 14345 |
| 2 | 14644 | 2 | 14883 |
| 3 | 14756 | 3 | 14929 |
| 4 | 14878 | 4 | 14943 |
| 5 | 14985 | 5 | 15245 |
| 6 | 15181 | 6 | 15177 |
| Mean | 14838.83 | Mean | 14920.33 |
| ± S. D. | 202.9839 | ± S. D. | 289.9073 |
| % R.S.D.(= 2) | 1.367924 | % R.S.D (= 2) | 1.943035 |
Table 8: Intermediate Precision for Clobetasol Propionate.
System precision: System precision was performed by the repetitive analysis of single homogenous solution of 100% test concentration 0.40 ug/mL and 10 ug/mL of Clobetasol Propionate and Luliconazole using the same instrument conditions as shown in Figures 5 and 6.
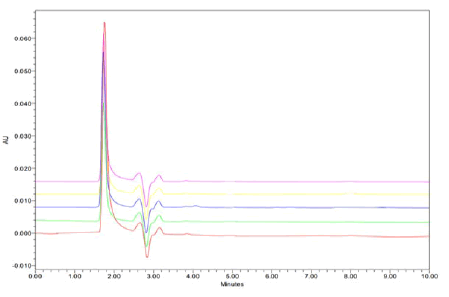
Figure 5: Linearity and Range Chromatogram of Luliconazole with Retention time 1.87min.
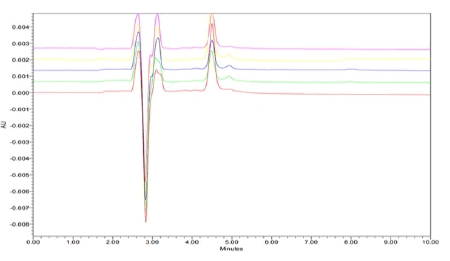
Figure 6: Linearity and Range Chromatogram of Clobetasol Propionate with retention time 4.35 min.
Method precision: The method Precision was performed by utilizing six replicate preparation of 100% test concentration of Clobetasol Propionate and Luliconazole. The percentage R.S.D of area for six repetitive chromatograms of Luliconazole and Clobetasol Propionate 100% test concentration for system precision and the area of six replicates of 100% test concentration for method precision are found <2% are shown in the Tables 9 and 10.
| Conc. 10 µg/ml | System precision Area | Conc. 10 µg/ml | Method Precision Area |
|---|---|---|---|
| 1 | 293080 | 1 | 292456 |
| 2 | 293145 | 2 | 292674 |
| 3 | 293297 | 3 | 292823 |
| 4 | 293387 | 4 | 293120 |
| 5 | 293467 | 5 | 293217 |
| 6 | 293587 | 6 | 293345 |
| Mean | 293543.8 | Mean | 292939.2 |
| ± S. D. | 446.7909 | ± S. D. | 314.0729 |
| % R.S.D (=1) | 0.152206 | % R.S.D (=2) | 0.107214 |
Table 9: Method Precision for Luliconazole.
| Conc. 0.50 µg/ml | System precision Area | Conc. 0.50 µg/ml | Method Precision Area |
|---|---|---|---|
| 1 | 14522 | 1 | 14345 |
| 2 | 14687 | 2 | 14483 |
| 3 | 14724 | 3 | 14829 |
| 4 | 14835 | 4 | 14943 |
| 5 | 14945 | 5 | 15045 |
| 6 | 15170 | 6 | 15170 |
| Mean | 14813.83 | Mean | 14802.5 |
| ± S. D. | 205.6821 | ± S. D. | 295.9987 |
| % R.S.D.(=1) | 1.388446 | % R.S.D (=2) | 1.999654 |
Table 10: Method Precision for Clobetasol Propionate.
For Clobetasol Propionate
The LOD was performed based on the standard deviation of the response and the slope
LOD=3.3* σ/S
Where σ=the standard deviation of the response
S=the slope of the calibration curve
The LOD of the Clobetasol Propionate was found to be 0.013 ug/mL
For the Luliconazole
It was also based on the Standard Deviation of the Response and the Slope
LOD=3.3 σ/S
Where σ=the standard deviation of the response
S=the slope of the calibration curve
The LOD of Luliconazole was found to be 0.269 ug/mL
Limit of Quantification
Limit of Quantification
LOQ of the Clobetasol Propionate
Likewise, the LOQ was performed based on the Standard Deviation of the response and the slope
LOQ=10* σ/S
Where σ=the standard deviation of the response
S=the slope of the calibration curve
The LOQ of Clobetasol Propionate was found to be 0.018 ug/ mL
LOQ of Luliconazole
Based on the Standard Deviation of the Response and the Slope
LOQ=10 σ/S
Where σ=the standard deviation of the response
S=the slope of the calibration curve
The LOQ of Luliconazole was found to be 0.817 ug/mL
A measure of an effective analytical method is how well its performance stands up to less than perfect implementation. Robustness can be done by any deliberate change in the procedure such as change in flow rate, change in pH, Effect of Change in wavelength (± 2 nm) and observing, and the effect over the output of the method as shown in Figure 7. The effect of change in flow rate, change in pH of mobile phase, Effect of Change in wavelength (± 2 nm) is shown in Tables 11 and 12.
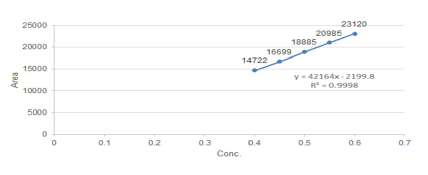
Figure 7: Linear Graph of Clobetasol Propionate with R2= 0.9998
| Sr. No. | Conc. (µg/mL) | Flow (1ml/min) | pH | Effect of Change in wavelength (± 2nm) |
|||
|---|---|---|---|---|---|---|---|
| 0.9 | 1.1 | 3.5 | 4 | 249 | 255 | ||
| 1 | 10 | 285656 | 304167 | 285774 | 294567 | 313457 | 311445 |
| 2 | 10 | 285759 | 304167 | 287978 | 294676 | 314354 | 315568 |
| 3 | 10 | 295850 | 304985 | 299113 | 296456 | 315456 | 317895 |
| Mean | 289088.3 | 3045 | 290955 | 295233 | 314422.3 | 314969.3 | |
| S.D | 4781.405 | 337.6 | 5838.329 | 865.9357 | 817.5175 | 2667.012 | |
| %RSD | 1.653 | 0.110 | 2.00 | 0.293 | 0.260 | 0.846 | |
Table 11: Robustness of Luliconazole.
| Sr. No. | Conc. (µg/mL) | Flow (1ml/min) | pH | Effect of Change in wavelength (± 2nm) | |||
|---|---|---|---|---|---|---|---|
| 0.9 | 1.1 | 3.5 | 4 | 249 | 255 | ||
| 1 | 0.40 | 14722 | 25820 | 15234 | 12134 | 15135 | 15345 |
| 2 | 0.40 | 14740 | 25837 | 15578 | 12157 | 15142 | 15350 |
| 3 | 0.40 | 14786 | 25846 | 14856 | 12123 | 15150 | 15360 |
| Mean | 14749 | 25834 | 15556 | 12138 | 15142 | 15351 | |
| S.D | 26.948 | 10.789 | 254.4 | 14.16 | 6.128 | 6.23 | |
| %RSD | 0.182 | 0.041 | 1.63 | 0.116 | 0.040 | 0.040 | |
Table 12: Robustness of Clobetasol Propionate.
Based on the results, this study is a typical example of the development of an assay method following ICH guidelines. A new isocratic RP-HPLC method has been developed and validated for determination of Luliconazole and Clobetasol Propionate in the pharmaceutical topical formulation. The results of the validation studies showed that the RP-HPLC method possesses significant linearity, precision, accuracy, specificity, sensitivity, high efficiency and resolution, and no interference from the excipients, as were demonstrated. The proposed method was successfully applied and is suggested for the quantitative analysis of Luliconazole and Clobetasol Propionate in combined pharmaceutical topical formulations for QC, where economy and time are essential and to assure therapeutic efficacy.
Received: 02-Aug-2021 Accepted: 16-Aug-2021 Published: 23-Aug-2021 , DOI: 10.35248/2157-7064.21.12.446
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.