Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2013) Volume 4, Issue 3
To separate resveratrol and emodin, supercritical carbon dioxide with ethanol was employed as the desorbent for a three-section simulated moving bed with an open-loop design called SF-SMB. Experimental validation of the separation was accomplished in this work. Silica was used as the stationary phase, and a crude extract of Polygonum cuspidatum containing mainly the resveratrol and the emodin, was purchased from a bio-technology company and used as the feedstock for the SF-SMB. Using single column chromatography, the operating conditions for a series of experiments conducted with 18 wt% ethanol were examined. The results were then compared to those predicted by the Triangle theory to determine the separable operating conditions and the dead volume of the SF-SMB unit. The robust operation of the SF-SMB against the concentration fluctuation of ethanol was also examined by conducting experiments with varied ethanol concentrations in different sections. It was found that the system is able to retain robust operation with about a 1.0 wt% ethanol fluctuation. Lowering the ethanol concentration to 15 wt% for each section was expected to relocate the separable operating conditions. A series of experiments with 12 wt% ethanol showed no pure raffinate. This is explained by the fact that the high flow rate of desorbent in the first section needed to obtain pure extract and raffinate would result in a high pressure drop, and lower the efficiency of the SF-SMB. From this study, the SF-SMB is demonstrated as being a useful technology for the separation of natural products, while providing a potentially greener alternative in the development of botanical drugs.
Keywords: Supercritical fluid-simulated moving bed chromatography; Resveratrol; Emodin
Resveratrol is recognized as an active compound extracted mainly from Polygonum cuspidatum and responsible for antibacterial, antioxidantive, anti-virus, and antitumor activity [1-4]. The crude extract of resveratrol from Polygonum cuspidatum also contains large amount of emodin, another bioactive compound. Both have the potential to be developed as botanical drugs so separation and purification of resveratrol and emodin has become a research focus [5-9]. In this work, the separation of resveratrol and emodin was conducted using a novel technology known as supercritical fluidsimulated moving bed (SF-SMB).
A simulated moving bed refers to a continuous chromatography, originally developed and applied in the separation of petrochemicals and sugars [10-12]. Two decades ago, SMB technology was scaled down and applied successfully in the pharmaceutical industry. In Asia, several crude extracts from natural products have also been successfully concentrated or purified using SMB chromatography [8,9,13-15]. If compared with batch chromatography, SMB is normally recognized as a green process in the pharmaceutical industry because of its low solvent and, energy use and water consumption [16]. However, dilution of the feed stream by the desorbent is still inevitable when employing SMB separation. In order to further reduce solvent and energy consumption, the liquid desorbent can be replaced by supercritical carbon dioxide [17-24].
Clavier et al. first applied supercritical carbon dioxide as the desorbent to SMB for the separation of GAL (g-linolenic ethyl ester) and DHA (docosahexaenoic ethyl ester). Later, researchers from TUHH (Hamburg University of Technology, Germany) and ETH (Swiss Federal Institute of Technology, Zurich, Switzerland) applied SFSMB in separating steroisomers, enantiomers and fatty acid ethyl esters [19-24]. The advantages of using a supercritical fluid as the desorbent include: instant evaporation of carbon dioxide to obtain concentrated products, the adjustment of solvent power by pressure, and the gradient operation of SMB attained by setting different pressures in different sections of the SMB. However, pure carbon dioxide is seldom used on its own as the eluent because of the injection of the feedstocks by a HPLC pump and the requirement of cosolvents in creating higher solubility and selectivity for the SF-SMB [17,19-24]. Therefore, the elution power can also be changed by the concentration of the cosolvent. The gradient operation of the SMB is also feasible by creating a concentration gradient along the SMB. Normally, the concentration of cosolvents significantly affects the retention of solutes. For robust operation, it therefore becomes crucial in controlling the cosolvent concentration.
In a four-section SMB, the fourth section is designed to regenerate the desorbent for recycling. Since carbon dioxide can be easily and instantly recycled by vaporization, the fourth section can be eliminated to enhance the efficiency of the SMB. Therefore, for this study a threesection SMB was designed for the SF-SMB, as illustrated in Figure 1. To apply supercritical carbon dioxide as the desorbent, a more complicated piping design was required; the design and operation were published in prior work [17]. In this work, experimental validation of the separation of resveratrol and emodin from the natural extract was conducted and the Triangle theory was applied to identify the separable range of the operation conditions. Since the solvent power of the supercritical fluid significantly changes with the concentration of ethanol, this study also investigated the effect of changing the concentration along the six-column to better understand its operation with varying ethanol concentrations.
The crude extract purchased from Baoji Hongyuan Bio-technology Co., Ltd. was submitted to HPLC analysis, and the dissolution of the crude extract into ethanol was used as the feed for the SF-SMB. About 10 g of the purchased extract was dissolved into 1.0 L of ethanol (95 vol%). After filtration, the clear solution contained 10,135 mg/L of solid.
A Syncronis C18 (Syncronis 97105-154360), 150×4.6 mm, and 5 μm from Thermo were used and 1.0 mL/min of the mixture of 80/20(V/V) of acetonitrile/water titrated by phosphoric acid to a pH ranging from 3.5~3.8, was used as the mobile phase. The injection volume is 20 μL. The isocratic elution was used for the analysis of all experimental results on the effluent of SF-SMB. A gradient elution set as Table 1 was also applied to identify major components and impurities in the crude extract. By the gradient elution, the spectrums at 280 nm for the feed and the effluent produced after the separation of SF-SMB were illustrated in Table 1 and Figure 2. The spectrum of the feed solution revealed that resveratrol and emodin were the major components of the crude extract and physcion was a trace impurity. By the calibration curves, the content of the resveratrol and emodin in the feedstock solution were found to be 8,524 and 996 mg/L, respectively. It was also found that the spectrums indicated at 280 nm had an area ratio of resveratrol to emodin near 8524/9966. Therefore the area of HPLC spectrums indicated at 280 nm was used as the concentration to calculate the purity and recovery of the effluent from the SF-SMB. The purity and recovery for the extract and raffinate of the SF-SMB were then calculated as:
| Time (min) | 0 | 15 | 25 | 30 | 50 |
| Acetonitrile | 15% | 40% | 50% | 60% | 100% |
| 0.1%H3PO4 | 85% | 60% | 50% | 40% | 0 |
Table 1: Setting for the gradient elution.
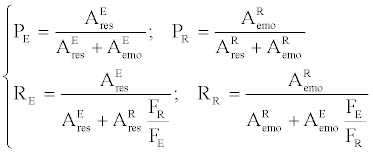 (1)
(1)
where P, R and F are the purity, the recovery, and the ethanol flow rates, and the subscripts of E and R represent the extract and raffinate, respectively. A denotes the area from the HPLC spectrums with 280 nm of UV wavelength, and the subscripts of res and emo represent resveratrol and emodin, respectively.
Single column chromatography by SFC
To conduct separation by SF-SMB, a single column studied by SFC (supercritical fluid chromatography) was carried out. Merck silica gel 60, 40~63 μm, was packed into a 150×10 mm column using the dry method. It was found that ethanol as cosolvent can separate resveratrol and emodin, and the concentration of ethanol significantly affects retention. Figure 2 illustrates the spectrums eluted by 12 and 18 wt% of ethanol and conducted at 40°C, 19.3 MPa, and 5.07 g-CO2/min. The injection volume is also 20 μL. It is noted that the retention of resveratrol and emodin are reversed, as compared to the liquid HPLC shown in Figure 3. From the spectrum with 12 wt% of ethanol, resveratrol is recognized as the stronger retention component and emodin as the weaker retention component. The impurities are hardly recognized by the SFC with 280 nm of UV wavelength. By increasing the weight percent of ethanol from 12 to 18 wt%, the retention time of resveratrol and emodin is largely reduced from 16.6 to 5.6 min and 10.3 to 3.5 min, respectively. Since the retention time for the non-retained solute was 1.0 min and the porosity of the packed column was 0.675, the Henry’s constants for the adsorption of resveratrol and emodin at 12 wt% were 32.4 and 19.3 respectively, and those at 18 wt% were 9.6 and 5.2. Although the Henry’s constant changed, the selectivity remained at roughly 1.7~1.8 without any change resulting from the ethanol concentration.
Operation of the SF-SMB
An additional six columns, 150×10 mm, were packed for the SF-SMB and installed according to the column porosity in the order of 0.653, 0.771, 0.748, 0.770, 0.763 and 0.765. In this study, the carbon dioxide flow rates for the feed, extract and raffinate were measured and controlled, and the flow rate of the desorbent was calculated by mass conservation. Ethanol was pumped into the SF-SMB from the desorbent and the feed streams by two independent HPLC pumps and discharged from the extract and raffinate along with the carbon dioxide. The weight fraction of ethanol for the extract and raffinate could then be evaluated by the mass conservation of the ethanol. As illustrated in Figure 1, the ethanol weight fraction of the extract, wEtOHE, should be equal to that of the desorbent, wEtOHD, and the mass conservation of the ethanol can then be used to evaluate the weight fraction of ethanol for the raffinate, wEtOHR. Because the content of solutes in the fluid phase is usually low, it can be neglected when calculating the mass flow rates of ethanol for the raffinate and the extract.
The Triangle theory is the theory most commonly used in setting the operating conditions for SMB. From the mass conservation, the Triangle theory predicts the relative volumetric flow rate in each section, following the constraints:
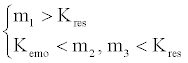 (2)
(2)
where Kres and Kemo are the Henry’s constant for resveratrol and emodin, and mj is the relative volumetric flow rate in section j. Accordingly, a right triangle on the (m2, m3) plane is usually drawn to define the SMB setting, and the coordinates of the plane represent the volumetric flow rate of the liquid desorbent relative to that of the solid phase in sections 2 and 3 of the SMB, respectively. Using the mass flow rate to replace volumetric flow rate when applying the Triangle theory to the SF-SMB has been proposed [22,24]. Although the expression by mass flow rate is much clearer and more easily understood, an estimate of the density of the supercritical fluid must still be calculated. In this study, relative volumetric flow rates were still used to determine the operating conditions and to explain the experimental results. In order to calculate the volumetric flow rate, the density of the supercritical fluid was calculated by the Peng-Robinson EOS (equation of state) without considering contributions from the solutes. The mixing rule and interaction parameters between carbon dioxide and ethanol have been cited directly from the literature [25].
If the dead volume of the SMB unit is considered, the relative volumetric flow rate in each section can be calculated as [26]:
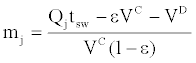 (3)
(3)
where VD is the estimated dead volume of the SF-SMB; VC is the empty
column volume, 7.845 mL; ε is the average porosity of the six columns, 0.745; tsw is the switching time of the valve; and Qj is the volumetric flow rate of the supercritical fluid in section j.
The separable operating conditions at 18 wt% ethanol
The separation of resveratrol and emodin by SF-SMB was conducted at 40°C and 19.3 MPa with ethanol controlled at 18 wt%. Typical spectrums at 280 nm for both extract and raffinate from the experiments are illustrated in Figure 4. In the experiment, the flow rates of the SF-SMB were fixed and the switching time of the valve varied, as listed in Table 2. The flow rate of carbon dioxide at desorbent and the flow rates of ethanol at extract and raffinate were evaluated by mass conservation. The recovery and purity were calculated by Equation (1), and the relative volumetric flow rates were derived using Equation (3).
| tsw(min) | m1 | m2 | m3 | Purity | Recovery | Remark | ||
|---|---|---|---|---|---|---|---|---|
| E | R | E | R | |||||
| 5 | 15.42 | 3.70 | 5.05 | 0.997 | 0.347 | 0.821 | 0.971 | Pure E |
| 5.5 | 17.34 | 4.45 | 5.93 | 0.997 | 0.335 | 0.807 | 0.978 | Pure E |
| 6.0 | 19.56 | 5.20 | 6.82 | 0.998 | 0.737 | 0.970 | 0.977 | Pure E & R |
| 6.5 | 21.18 | 5.95 | 7.70 | 0.997 | 0.767 | 0.961 | 0.981 | Pure E & R |
| 7.5 | 25.02 | 7.45 | 9.47 | 0.997 | 0.770 | 0.969 | 0.969 | Pure E & R |
T=40°C; P=19.3 MPa; Density of SF=925 kg/m3
DCO2=5.705* g/min; DEtOH=1.255 g/min; FCO2=0.400 g/min; FEtOH=0.088 g/min;
ECO2=3.480 g/min; EEtOH=0.766* g/min; RCO2=2.630 g/min; REtOH=0.577* g/min
Table 2: Operating conditions and experimental results for SF-SMB.
The experimental setting of the switching time was first estimated by the Triangle theory without considering the dead volume. After comparing the separable operating conditions from the experimental results with those of the Triangle theory, the dead volume of the SF-SMB was estimated as 1.8 mL. The illustration of the Triangle theory on the (m2, m3) plane is shown in Figure 5. From Table 2, it is observed that the experimental results with switching time ranged from 6.0~7.5 min could successfully separate the resveratrol and emodin, and the right triangle in Figure 5 could be used to define the boundary of the separable region, and the apex of the right triangle represents optimum for the SF-SMB at 19.3 MPa, 40°C, and 18 wt% of ethanol.
Effects of ethanol concentration on separation
As shown in Figure 3, the elution of the resveratrol and emodin is significantly affected by the ethanol content. The fluctuation of ethanol concentration may seriously affect the separation and lead to a failure of the robust operation of the SF-SMB. An additional four experiments were conducted to investigate the robust operation of the SF-SMB in regard to the change of concentration. The switching time of the four experiments was fixed at 6.5 min with flow rates as listed in Table 3. The calculated relative volumetric flow rates and the purity and recovery for each test are listed in Table 4. In Table 4, the number in parenthesis represents the weight percent of ethanol in each section. From the test runs of 6 and 7, it is observed that the SF-SMB can effectively separate the resveratrol and emodin when a fluctuation in the concentration of ethanol is within about 1.0 wt%. In both tests, the operating gradients in ethanol concentration also had possible effects on the stability of the operation.
| Flow Rate (g/min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Run 6 | Run 7 | Run 8 | Run 9 | |||||
| CO2 | EtOH | CO2 | EtOH | CO2 | EtOH | CO2 | EtOH | |
| Desorbent | 5.705 | 1.255 | 5.705 | 1.432 | 4.015 | 1.005 | 5.705 | 1.009 |
| Feed | 0.400 | 0.055 | 0.400 | 0.055 | 0.400 | 0.055 | 0.400 | 0.055 |
| Extract | 3.475 | 0.766 | 3.475 | 0.874 | 1.785 | 0.447 | 3.475 | 0.615 |
| Raffinate | 2.630 | 0.544 | 2.630 | 0.613 | 2.630 | 0.613 | 2.630 | 0.449 |
Table 3: Setting of the flow rates for the test of concentration fluctuation.
| Run | m1 | m2 | m3 | Purity | Recovery | Remark | ||
|---|---|---|---|---|---|---|---|---|
| E | R | E | R | |||||
| 6 | 20.66 | 5.77 (18.0%) |
7.39 (17.1) |
0.997 | 0.820 | 0.962 | 0.982 | Pure E & R |
| 7 | 21.26 | 6.00 (20.1%) |
7.60 (18.9%) |
0.997 | 0.751 | 0.961 | 0.973 | Pure E & R |
| 8 | 13.83 | 6.00 (20.0%) |
7.60 (18.9%) |
0.997 | 0.646 | 0.970 | 0.952 | Pure E & R |
| 9 | 19.93 | 5.48 (15.0%) |
7.10 (14.6%) |
0.995 | 0.224 | 0.556 | 0.978 | Pure E |
Table 4: The experimental results for the test of concentration fluctuation.
It is also noted that the relative volumetric flow rate in the first section for test runs 6 and 7 was much higher than the Henry’s constant of resveratrol. It is, therefore, possible to decrease m1 to reduce the solvent and energy consumption for the downstream concentration. Test run 8 was operated at m1=13.83, which still fulfilled the constraint as in Equation (2) even though it was much lower than it was for runs 6 and 7. Therefore, the separation of resveratrol and emodin was still achieved, although the purity of emodin was slightly reduced. This implies that part of the resveratrol was entrained by the recycled solid and contaminated the raffinate.
When the ethanol concentration was decreased to about 15 wt% as indicated by test run 9, the separation failed. From the spectrums shown in Figure 3, it is expected that the separable operating conditions for 15 wt% of ethanol should be largely different from those of 18 wt%. This suggests that the right triangle defined in Figure 5 for 18 wt% of ethanol is no longer suitable for that for 15 wt%. The triangle needs to be relocated for the operation at 15 wt%.
A series of experiments conducted at 12 wt% of ethanol in this study also failed to separate the resveratrol and emodin because the SF-SMB failed to increase the relative volumetric flow rate in the first section to above 32.4, which is the Henry’s constant of resveratrol at 12 wt%. Although it is observed from Figure 3 that the resolution of the resveratrol and emodin at 12 wt% of ethanol is much higher than that at 18 wt%, the requirement of a large relative volumetric flow rate in the first section of SMB causes an increased drop in the pressure of the SF-SMB. It is concluded that the operation of the SMB is determined mainly by the selectivity rather by the resolution.
In this work, ethanol was used as a cosolvent for the supercritical carbon dioxide in order to elute the resveratrol and emodin from silica. A novel unit of supercritical fluid in a simulated moving bed was used to realize the continuous operation of the elution needed to separate the resveratrol and emodin. The separable operating conditions of the SF-SMB were identified by comparing the experimental results with the Triangle theory for 18 wt% of ethanol. Also the robust operation related to the ethanol concentration was experimentally evaluated as being about 1.0 wt%. It is also concluded that the SF-SMB system would become inefficient if operated at 12 wt% of ethanol. The SF-SMB is a useful technology for purifying natural products, and more advantages and applications of the SF-SMB still await discovery.
Grant Supports from the National Science Council and the Institute of Nuclear Energy of Taiwan are acknowledged.