Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2016) Volume 7, Issue 1
Herein we discuss physical properties of 4-(ω-hydroxyalkoxy)-4’-cyanoazoxybenzene homologs. 1D and 2D correlation NMR spectroscopy (in particular, 1Ð�, 15N-HMBC experiment) have allowed elucidation of structure of the prepared rod-like supramolecular cyanoazoxybenzenes. Mesomorphic properties of the compounds have been studied by means of polarization thermomicroscopy and differential scanning calorimetry. All the studied cyanoazoxybenzenes have revealed enantiotropic nematic mesomorphism over wide temperature range. Nematic mesophase of the eighth homolog has possessed large positive dielectric anisotropy. Introduction of small amounts of the prepared cyanoazoxybenzenes as additive has stabilized the mesophase and has increased the dielectric anisotropy of 4-pentyloxy-4’-cyanobiphenyl. Gas-liquid chromatography studies have shown that sorbents based on 4-(2-hydroxyethyloxy)-4’-cyanoazoxybenzene are highly selective towards various structural isomers; that cannot be achieved using conventional nematic liquid crystals. Thermodynamic evidence of specific interactions between the mesogen and the non-mesomorphic sorbate has been discovered.
Keywords: Mesogen; Azoxybenzene; Hydrogen bond; Position isomerism; NMR spectroscopy; Nematic phase; Supramolecule; Dielectric anisotropy; Double refraction; Sorbent; Structural selectivity
Modern supramolecularchemistry is among most dynamically developing branches of science [1-5]. It includes study of chemical, physical, biological, and other aspects of complex chemical systems linked together via intermolecular (non-covalent) interactions. Hydrogen bond occupies a special place among diverse non-covalent interactions (van der Waals and donor-acceptor ones, coordination bonding involving metal ions, etc.). Being stereospecific, relatively strong, and dynamic, hydrogen bond has been recognized as key interaction in supramolecular systems [6].
Intermolecular hydrogen bonds are crucially important in formation of liquid-crystalline materials as well, as supramolecular selfassembly may result in emergence of novel properties: phase transitions, photoinduced effects, conductivity, proton transport, etc. In view of this, liquid crystal systems linked via hydrogen bonds are considered typical objects of supramolecular chemistry, as mesomorphism can be discussed regarding a sufficiently populated supramolecular assembly [7-9].
Liquid-crystalline molecular associates may be formed either of identical molecules (Figures 1a-c) or of chemically different compounds (Figure 1d).
Studies of Bennet [10] and Gray [11] pioneered in study of liquid crystals based on aromatic carboxylic acids and cinnamic acid; such materials exhibit mesomorphism due to formation of cyclic dimers linked via hydrogen bonds (Figure 1a). Mesogenic dimer is the simplest assembled form of supramolecular liquid crystals, the most widespread, and the best studied [12-15]. Amides are known to form supramolecular chains via hydrogen bonding [16] (Figure 1b), whereas polyhydric alcohols (sugars [17-20], hexahydroxycyclohexane [21], and diols [22-24]) may form layered structures (Figure 1c).
Supramolecular mesogens formed via the molecular recognition of involving various compounds are by far more numerous [25,26] (Figure 1d). Such binary systems may consist of two liquid-crystalline [27,28], two non-mesomorphic [29], or a liquid-crystalline and a nonmesomorphic [30,31] components. Noteworthily, azaheterocyclic compounds (derivatives of pyridine, azopyridine, and 4,4’-bipyridine) have been recognized as the most promising non-mesogenic building blocks [32-38]. In all the cases formation of strong hydrogen-bound complex are reflected by special properties of a liquid-crystalline product, distinct from these of the starting components.
Our group has contributed to investigation of liquid-crystalline systems involving hydrogen bonds as well [39,40]. We have extended the range of conventional reactive polar substituents constituting the discussed systems by synthesis study of mesogenic derivatives of azobenzene, benzylideneaniline, phenylbenzoate, and biphenyls containing aldehyde, aldoxime, epoxy, and other terminal groups [41,42]. The self-assembly via hydrogen bonding to form polymeric supramolecular assemblies was aided by the prepared bifunctional mesogenic compounds bearing at least two specifically interacting complementary fragments in the molecule [43-45]. We ruled out certain regularities of the self-assembly effect on mesomorphic, volume, rheological, dielectric, orientation, and sorption properties of the functional liquid crystals [46-49]. We generalized the most significant results of our studies and denoted the promising fields of practical applications of supramolecular mesogenic structures [50].
Analysis of the available reference literature on supramolecular liquid crystals has revealed that majority of the published reports have discussed preparation and formation conditions of supramolecular mesophases as well as phase diagrams of binary mixtures of the complementary components, identification of the mesophases, and variation of phase transition temperature. However, the information on the effect of specific interactions on physical properties of supramolecular liquid crystals has been scarce so far.
The azoxybenzenes class is of significant fundamental and applied importance among organic mesomorphic substances. Some of their properties, including high thermal stability, wide temperature range of mesomorphism, good miscibility with other mesogens, and sufficiently low viscosity make them promising materials for nonlinear optics [51- 53] and gas chromatography [54] applications.
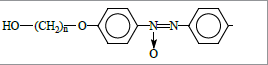
In this work we present the studies of physical properties of compounds containing two benzene rings bridged by azoxy group as a molecule core, the end groups being -CN and -ROH (R stands for hydrocarbon spacer) (Figure 2); possible practical applications of these compounds are discussed as well.
A special feature of the discussed compounds is the presence of two polar reactive substituents, allowing for further chemical modification to give functional materials with desired mesogenic properties as well as those based on macroheterocycles suitable for advanced technical and scientific applications.
In particular, the interaction of epichlorohydrin and epoxy function allows introduction of the oxirane heterocycle at the aliphatic substituent of cyanoazoxybenzenes Ia-e. Such mesogens may be efficient stabilizers in compositions based on thermoplastic polymers.
Introduction of the terminal acryloyl moiety via interaction of the studied azoxybenzenes Ia-e with acryloyl chloride results in formation of fairly promising monomers suitable for development of smart lightcontrolled liquid-crystalline polymers that may be used for reversible or irreversible black-and-white or color information recording and storage, optical memory systems, display technology, optoelectronics, holography, etc.
Crown ether-containing monomers based on reactive azoxybenzenes Ia-e allow preparation of multi-functional photochromic-ionophore liquid-crystalline copolymers for development of self-assembled photo-controlled sensor devices. On the other hand, hydrolysis of the -CN group yields carboxylic acids, the corresponding acyl chlorides being capable of the reactions with reactive substituents of porphyrins and phthalocyanines, thus opening vast opportunities for structure modification of macroheterocyclic compounds to provide various functional materials.
Preparation, modification and mesomorphic properties
Preparation of 4-(ω-hydroxyalkyloxy)-4’-cyanoazoxybenzenes was described elsewhere [55]. The mesogens were purified by recrystallization from ethanol followed by incubation under residual pressure of 200 Pa at the temperature of mesophase existence during 12 h. The sufficient purity of the prepared specimens was judged by the absence of the impurities signals in the NMR spectra, the constant temperature of nematic-isotropic phase transition (TNI) after repeated purification steps, and no disintegration into the nematic (N) and isotropic (I) phases in the course of the phase transition.
1H, 13C, and 15N NMR spectra were registered using a Bruker Avance III-500 instrument in CDCl3 at 35°C. The carbon (δC=77.00 ppm) and residual proton (δH=7.27 ppm) signals of the solvent and liquid ammonia (δN=0.0 ppm) were used as internal and external references, respectively. All the experiments were run according to the manufacturer recommendation. The evolution time in ???? 1H-13C and 1H-15N experiments was of 60 and 125 ms, respectively.
Phase transition temperatures of the individual liquid crystals and the binary mesogens systems were measured using polarization thermomicroscopy (PTM) and differential scanning calorimetry (DSC) techniques. The determined temperatures were further checked during gas-liquid chromatography and dielectric constant measurement experiments.
The PTM studies were performed using a Polam L211 polarization microscope equipped with a temperature stage allowing heating at 0.1-3.5 deg/min rate over a wide temperature range of 0-500°C as well as prolonged incubation of a specimen at a desired temperature. The accuracy of temperature reading was of ± 0.1°C.
DSC curves were recorded using a DSC 204 F1 Phoenix calorimeter; the heating and cooling experiments were performed at 30-350°C at the rate of 5 deg/min.
The mixtures of 4-pentyloxy-4’-cyanobiphenyls (5OCB) with Ia-e were prepared by weighing using a ?? 20 (accuracy of 0.05 mg) and Sartorius genius ME215 P (0.01 mg) balances. Liquid-crystalline substance 5OCB (Aldrich) was used as received.
Dielectric properties and birefringence
Static dielectric constants of compound Ic and nematic binary mixtures in the mesomorphic and isotropic liquid states was measured taking advantage of dielcometric technique using a constanttemperature cell consisting of two parallel 19.6 mm2 plates separated by a 0.25 mm gap filled with a tested substance. The specimen orientation was aided by a 2000 G electromagnet. The cell capacitance was measured with a LCR-817 (INSTEK) instrument at 1000 Hz and 1.2 V. The cell was calibrated using reference compounds with known dielectric constant. The dielectric constant was calculated as follows:
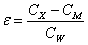
with CX being capacitance of the cell with the studied specimen; CM being wiring capacitance as determined from the cell calibration; and CW being capacitance of the cell working distance from the calibration.
In order to estimate the dielectric anisotropy 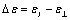 we performed the measurements parallel
we performed the measurements parallel 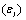 and perpendicular
and perpendicular 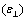 to the nematic liquid crystal director, over a range of temperatures set up using an UH-16 thermostat. The relative error of dielectric constant measurement was of 1%, Δε calculated using statistics methods was of 3%.
to the nematic liquid crystal director, over a range of temperatures set up using an UH-16 thermostat. The relative error of dielectric constant measurement was of 1%, Δε calculated using statistics methods was of 3%.
Birefringence of a liquid-crystalline materials was expressed as 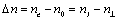 Refractive indexes of ordinary ray in the mesomorphic state
Refractive indexes of ordinary ray in the mesomorphic state 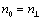 and in the isotropic phase nis were determined using a constant-temperature Abbe refractometer at 589 nm with accuracy of ± 0.0005. Surface of the refractometer prisms was rubbed in order to facilitate the specimen orientation. Refractive index of extraordinary ray
and in the isotropic phase nis were determined using a constant-temperature Abbe refractometer at 589 nm with accuracy of ± 0.0005. Surface of the refractometer prisms was rubbed in order to facilitate the specimen orientation. Refractive index of extraordinary ray 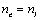 was calculated using the equation for the average value
was calculated using the equation for the average value 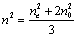 , the latter being determined via nis extrapolation to the range of nematic phase. The error of birefringence determination was of 0.5% (n) and 1.0% (?n).
, the latter being determined via nis extrapolation to the range of nematic phase. The error of birefringence determination was of 0.5% (n) and 1.0% (?n).
Gas-liquid chromatography
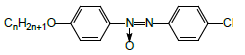
4,4’-Dimethoxyazoxybenzene LC-1 (Aldrich) was used as received. 4-Propyloxy-4’-cyanoazoxybenzene LC-2 was prepared as described elsewhere [56].
Mesogens LC-1, LC-2, and Ia (9.95 wt.%) were applied onto a solid stationary phase Chromaton N-AW (0.40-0.63) via evaporation of the chloroform solution. The so prepared sorbent was introduced into metal 1 m × 3 mm columns under vacuum. Each column was conditioned during 6 hr at the highest operating temperature.
Retention time of the sorbates (p- and m-xylene and 3- and 4-methylanisole as well as isomeric ????????? lutidins and picolines) was measured using a Shimadzu GC-2014 gas chromatograph equipped with a flame ionization detector, helium being the carrier gas. Shimadzu GC-solution Chromatography Data System Version 2.4 software allowed setting the column, evaporator, and detector temperatures (at 0-400°C with accuracy of ± 0.1°C) as well as feed and pressure of the carrier gas at the column input and output; the retention time was determined with accuracy of 0.5 s. An autosampler Shimadzu AOC-20i syringe Shimadzu (10 μL) was used to apply small volume of the sorbates (no more than 0.1 μL) to the column so that the experiment conditions matched the limiting dilution and the sorbate concentration was within the linear range of the dissolution isotherm. The dead (void) time was determined using propane as reference.
For physico-chemical parameters of the sorbates, the corresponding A, B, and C coefficients in the Antoine equation, and procedures of calculation of the sorbates saturated vapor pressure and thermodynamic sorption parameters [57].
Identification and mesomorphic properties
Structures of azoxybenzenes Ia-e were elucidated by means of NMR spectroscopy. The 1? NMR spectra contained the duplicated set of signals of para-disubstituted benzene rings (the ??’??’ system) pointing at formation of the isomers ? and ? differing in oxygen position under the preparation conditions. Integration of the 1? NMR spectra of samples Ia-e revealed that the ?/? ratio was almost equimolar (Figure 3).
The presence of nitrogen atom in the compounds Ia-e allowed elucidation of their structure by means of the 1H,15N-HMBC experiment optimized for the spin-spin coupling constant of 4 Hz revealing the 1?-15N interactions via several bonds. Noteworthily, the signals of cyano group nitrogen were not observed in the spectra when using the indirect detection of 15N, likely due to the low value of heteronuclear spin-spin interaction constant. However, the presence of -C≡N groups was confirmed by 13? NMR spectra containing characteristic signals at the 118 ppm region. On the other hand, appearance of the 15N signals at 310-330 Hz evidenced about formation of azoxy derivatives. It is known that the signal of the oxidized nitrogen atom (=NO-) of azoxybenzenes experienced an upfield shift in the 15N NMR spectra [58,59]. Hence, the structure of isomers A and B as well as their ratio could be elucidated from the available spectral data (Figure 4).
We attempted separation of isomers of supramolecular azoxybenzenes Ia-e via recrystallization from ethanol or benzene as well as via column chromatography on alumina (methylene chloride as eluent) or on silica gel (diethyl ether-chloroform-benzene-ethanolacetic acid 1:1:1:0.25:0.25 as eluent). However, the isomers ratio of compounds I was not changed upon the purification, likely due to the structural features. The presence of hydroxyl groups capable of strong specific interactions in compounds I seemed to level off the differences in the solvation and elution behavior originating from structure of the bridging groups Table 1.
| Compound | Isomers ratio | Phase transitions temperature,°С | Compound | Phase transitions temperature, °С | |||
|---|---|---|---|---|---|---|---|
| I-A | I-B | С→N | N→I | С→N | N→I | ||
 |
 |
||||||
| Ia(n=2) | 45 | 55 | 136.6 | 188.1 | n=2 | 169.5 | 179.0 |
| Ib (n=3) | 55 | 45 | 124.5 | 167.0 | n=3 | 127.5 | 156.5 |
| Ic (n=6) | 58 | 42 | 127.5 | 166.4 | n=6 | 114.0 | |
| Id (n=8) | 59 | 41 | 117.6 | 150.3 | n=8 | C70.8S120.4N133.7I | |
| Ie (n=10) | 59 | 41 | 111.3 | 138.0 | n=9 | C73.5S119.5N127.7I | |
Table 1: Temperature of phase transitions of azoxybenzenes I and their structural analogs (accuracy of ± 0.1°С).
Phase transitions temperature of 4-(ω-hydroxyalkyloxy)-4'- cyanoazoxybenzenes Ia-e and their structural analogs 4-alkyloxy- 4'-cyanoazoxybenzenes as determined by the PTM method are collected in Table 1. The tabulated data revealed significant influence of the terminal groups on the phase transitions temperature. First, the active substituent stabilized the nematic phase. In particular, whereas the higher homologs of “conventional” cyanoazoxybenzenes were smectic-nematic, the bifunctional azoxybenzenes Ia-e exhibited nematic mesomorphism even in the case of compound Ie with 10 carbon atoms in the aliphatic fragment. Moreover, introduction of the terminal hydroxyl group resulted in increase of the phase transitions temperature, the increase being more prominent for the nematicisotropic transition; hence, the temperature range of existence of the anisotropic phase was expanded. That could be due to appearance of sufficiently strong hydrogen bonds. Two types of interaction were possible: the chain-like “head to tail” association and the “tail to tail” dimerization (Figure 5). In the both cases the effective anisotropy of molecular polarizability was expected to increase, and the mesophase thermal stability should have been enhanced. Additionally, that should have resulted in significant restriction of the aliphatic fragments mobility and their higher orientation ordering.
DSC data demonstrated that energy of the nematic-isotropic phase transition of supramolecular azoxybenzenes Ia-e was of 2.64-2.87 kJ/ mol, significantly higher than that of the azoxybenzenes not containing terminal OH groups in the aliphatic fragment and hence not capable of the self-assembly via hydrogen bonding (0.85-1.37 kJ/mol). The high enthalpy of the phase transition from the ordered liquid-crystalline state into the disordered isotropic liquid was likely due to disruption of the intermolecular hydrogen bonds.
Anisotropic properties
Operation parameters of visual indication devices taking advantage of electrooptical effects of liquid crystals depend on anisotropy of dielectric properties of the mesogens. Therefore, measurement of components of dielectric permittivity tensor is of primary importance for development of new materials and estimation of their potential. In view of that, we studied static dielectric permittivity of 4-(8-hydroxyoctyloxy)-4'-cyanoazoxybenzene Id (Figure 6).
The studied compound Id revealed positive dielectric permittivity anisotropy 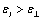 and sufficiently high dielectric constant value, due to the presence of highly polar cyano group in the terminal fragment. Phase transitions temperature determined using dielcometric method coincided well with those determined by thermomicroscopy.
and sufficiently high dielectric constant value, due to the presence of highly polar cyano group in the terminal fragment. Phase transitions temperature determined using dielcometric method coincided well with those determined by thermomicroscopy.
In the case of conventional 4-octyloxy-4’-cyanoazoxybenzene Δε=9.5 [56] at ?red=?-TNI=-7°?. Introduction of terminal hydroxyl group increased the ?? value up to 11.8 in the case of compound Id. That could be due to both the increase of overall dipole moment of the molecule upon addition of a polar hydroxyl group and significant changes of nature of the association processes in the nematic and isotropic phases of supramolecular azoxybenzenes as compared to their analogs not capable of self-assembly.
The high temperature of mesophase existence complicates the application of compounds I in pure form. In view of that, investigation of efficiency of bifunctional azoxybenzenes Ia-e as dopants of lowtemperature cyanobiphenyls is of definite interest. In particular, we studied the influence of compounds Ia-e on mesomorphic and anisotropic properties of their mixtures with 4-pentyloxy-4'- cyanobiphenyl (5OCB).
Figure 7 displays a part of phase diagram of the 5OCB + I? mixture and the values of slope of the phase boundary of the 5OCB + 4-(ω-hydroxyalkyloxy)-4'-cyanoazoxybenzene mixtures. We found that compounds Ia-e were fully miscible with 5OCB and stabilized the mesophase. The highest stabilizing effect was revealed in the case of compound I?, and compound Ie was the least efficient stabilizer; seemingly, the effect was determined by the azoxybenzenes geometry parameters.
We obtained temperature dependences of static dielectric permittivity and refractive indexes of the studied systems; they were recalculated into the data on dielectric anisotropy Δε and birefringence Δn, respectively (Figures 8 and 9). The temperature dependences of ?, n, Δε, and Δn were typical of mesomorphic materials; addition of hydroxyl-containing components Ic-e resulted in enhancement of dielectric anisotropy and birefringence.
Selectivity and thermodynamics of dissolution of structural isomers in liquid-crystalline azoxybenzenes
Doping of composite liquid-crystalline materials is not the only possible practical application of 4-(ω-hydroxyalkyloxy)-4'- cyanoazoxybenzenes Ia-e. Due to wide range of the mesophase existence and low viscosity, liquid-crystalline azoxybenzenes are widely used as stationary phases in gas chromatography for analytical separation of structural isomers of organic compounds [60]. 4,4'-Methoxyethoxyazoxybenzene (MEAB) exhibiting the factor of structural selectivity towards meta-para xylenes separation (1.13 in the nematic phase) is among the most selective liquid crystals for chromatography applications [61]. That allows separation of xylenes using a 1 m column, the operation being often impossible using 10-15 m conventional capillary columns. Such excellent selectivity is due to the absence of extensive regions of low ordering in the MEAB mesophase, the reason for the perfect ordering being the short and limitedly mobile aliphatic substituents (methyloxy and ethyloxy) of this mesogen. Hence, the orientation ordering over the liquid-crystalline sample volume and steric limitations for the separated sorbates are leveled off.
At the same time, the MEAB selectivity is not sufficient for gas chromatography separation of certain multicomponent mixtures. We have earlier demonstrated that taking advantage of supramolecular factor of structural selectivity allows for efficient analytical separation of xylene isomers as well as higher-boiling compounds: p- and m-cresols, p- and m-methylanisoles, 3,4- and 3,5-lutidins, etc. [62]. In view of that, MEAB analogs capable of self-assembly via the active terminal groups are of definite fundamental and applied interest.
Using the gas chromatography experiment data (retention times of pairs of structural isomers) we determined the highest coefficients of structural selectivity ? of a sorbent containing 4-(2-hydroxyethyloxy)- 4'-cyanoazoxybenzene Ia as stationary phase (Table 2).
| Isomers | Column temperature, °С | α |
|---|---|---|
| p-andm-xylene | 103.9 | 1.12 |
| p-andm-methylanisole | 105.6 | 1.1 |
| 3,4-and3,5-lutidin | 101.5 | 1.75 |
| p-and m-cresol | 139.8 | 1.03 |
Table 2: Highest coefficients of structural selectivity of compound Ia.
The experimental results demonstrated excellent selectivity of the sorbent based on 4-(2-hydroxyethyloxy)-4’-cyanoazoxybenzene Ia towards isomers of high-boiling organic compounds (α=1.75 in the case of 3,4- and 3,5-lutidins) and high selectivity towards separation of para- and meta-xylenes (α=1.12). Hence, mesogen Ia is a promising liquid-crystalline stationary phase to be used for quantitative analysis of mixtures of organic compounds [55].
Noteworthily, sorption behavior of isomeric lutidins was remarkably different from that of xylenes, methylanisoles, and cresols. In particular, the less anisotropic 3,4-lutidin revealed higher retention time as compared to the more anisotropic 3,5- isomer. Moreover, the separation coefficient of lutidins was higher than that of other sorbate pairs. Those peculiarities evidenced that specific interactions of the electron-donating sorbate and the proton-donating terminal group of the mesogen should be accounted for along with the steric separation factor in the case of sufficiently basic sorbates. Indeed, 3,4-lutidin incorporation in the stationary phase was favorable over the interaction of more anisotropic 3,5-lutidin:
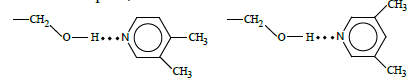
At the same time, the integral structural selectivity towards separation of pyridine derivatives could be affected by the following factors:
1. purely steric limitations;
2. selective dipole-dipole interactions;
3. selective specific interactions;
4. supramolecular effect.
In order to elucidate contributions of the above-listed factors, we studied dissolution selectivity and thermodynamics of electrondonating isomeric lutidins and picolines in the stationary phases based on the following nematic azoxybenzenes:
• low-polar “conventional” 4,4'-dimethoxyazoxybenzene LC-1
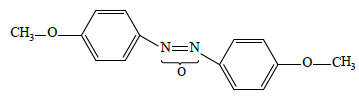
• high-polar “conventional” 4-propyloxy-4'-cyanoazoxybenzene LC-2
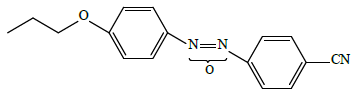
• supramolecular 4-(2-hydroxyethyloxy)-4'-cyanoazoxybenzene Ia
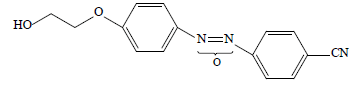
We calculated the Herington coefficients of structural selectivity from the gas chromatography experiment data (Table 3).
| Mesogen | Lutidins | Picolines | |||||
|---|---|---|---|---|---|---|---|
| α(2,5/2,6) | α(2,4/2,5) | α(2,3/2,4) | α(3,5/2,3) | α(3,4/3,5) | α(2/3) | α(3/4) | |
| LC-1 | 1.80 | 1.07 | 1.04 | 1.54 | 1.34 | 1.70 | 1.11 |
| LC-2 | 1.89 | 1.00 | 1.09 | 1.49 | 1.35 | 1.68 | 1.05 |
| Ia | 1.96 | 1.12 | 1.03 | 1.49 | 1.51 | 1.74 | 1.17 |
Table 3: Coefficients of structural selectivity of azoxybenzenes.
Supramolecular liquid-crystalline material Ia exhibited the highest selectivity. Decrease of the overall structural selectivity of LC-2 as compared to LC-1 was likely due to the increase of the terminal alkyl substituent length resulting in appearance of the regions of deteriorated orientation ordering.
We recalculated the retention experimental data into thermodynamic parameters of dissolution of isomeric lutidins and picolines (Tables 4-6).
| Sorbate | γ ∞ | –ΔH∞, kJ/mol | –ΔS∞, J/(mol·K) | HE, kJ/mol | SE, J/(mol·K) | GE, kJ/mol |
|---|---|---|---|---|---|---|
| T=401 K | ||||||
| 2,6-lutidin | 1.76 | 18.0 | 49.3 | 16.5 | 36.8 | 2.0 |
| 2,5-lutidin | 1.36 | 22.8 | 59.3 | 15.5 | 36.3 | 1.1 |
| 2,4-lutidin | 1.38 | 22.0 | 57.3 | 14.4 | 33.6 | 1.1 |
| 2,3-lutidin | 1.37 | 21.6 | 56.3 | 16.1 | 37.8 | 1.1 |
| 3,5-lutidin | 1.23 | 26.2 | 66.8 | 13.2 | 31.3 | 0.7 |
| 3,4-lutidin | 1.14 | 30.3 | 76.4 | 10.3 | 24.7 | 0.4 |
| 2-пиколин | 1.29 | 21.3 | 55.3 | 12.4 | 29.0 | 0.8 |
| 3-пиколин | 1.15 | 20.0 | 51.0 | 14.5 | 35.0 | 0.5 |
| 4-пиколин | 1.09 | 23.1 | 58.2 | 10.6 | 25.6 | 0.3 |
| T=413 K | ||||||
| 2,6-lutidin | 1.30 | 30.4 | 75.8 | 3.3 | 5.7 | 0.9 |
| 2,5-lutidin | 1.05 | 34.4 | 83.7 | 2.3 | 5.2 | 0.2 |
| 2,4-lutidin | 1.06 | 35.2 | 85.6 | 2.3 | 5.2 | 0.2 |
| 2,3-lutidin | 1.02 | 34.6 | 84.0 | 2.5 | 5.8 | 0.1 |
| 3,5-lutidin | 0.93 | 36.1 | 86.8 | 2.5 | 6.6 | -0.2 |
| 3,4-lutidin | 0.88 | 38.9 | 93.0 | 0.3 | 1.9 | -0.4 |
| 2-пиколин | 0.97 | 29.9 | 72.2 | 1.7 | 4.2 | -0.1 |
| 3-пиколин | 0.88 | 31.7 | 75.6 | 1.9 | 5.7 | -0.4 |
| 4-пиколин | 0.84 | 32.5 | 77.3 | 1.2 | 4.4 | -0.6 |
Table 4: Thermodynamic parameters of dissolution of substituted pyridines in the nematic and isotropic phases of LC-1 at infinite dilution.
| Sorbate | γ ∞ | –ΔH∞, kJ/mol | –ΔS∞, J/(mol·K) | HE, kJ/mol | SE, J/(mol·K) | GE, kJ/mol |
|---|---|---|---|---|---|---|
| T=408 K | ||||||
| 2,6-lutidin | 2.04 | 23.9 | 64.5 | 10.3 | 19.2 | 2.4 |
| 2,5-lutidin | 1.52 | 27.4 | 70.5 | 10.0 | 21.0 | 1.4 |
| 2,4-lutidin | 1.64 | 26.9 | 70.0 | 9.2 | 18.4 | 1.7 |
| 2,3-lutidin | 1.55 | 27.1 | 70.1 | 10.4 | 21.8 | 1.5 |
| 3,5-lutidin | 1.43 | 28.5 | 72.6 | 11.3 | 24.8 | 1.2 |
| 3,4-lutidin | 1.36 | 30.1 | 76.2 | 11.2 | 25.1 | 1.0 |
| 2-picoline | 1.47 | 23.4 | 60.5 | 8.8 | 18.3 | 1.3 |
| 3-picoline | 1.33 | 25.2 | 64.2 | 9.0 | 19.5 | 1.0 |
| 4-picoline | 1.32 | 25.4 | 64.5 | 9.0 | 19.7 | 0.9 |
| T=433.2 K | ||||||
| 2,6-lutidin | 1.56 | 31.4 | 77.1 | 1.2 | -1.7 | 1.6 |
| 2,5-lutidin | 1.20 | 32.2 | 75.9 | 2.8 | 5.1 | 0.7 |
| 2,4-lutidin | 1.30 | 32.5 | 76.6 | 2.8 | 5.1 | 0.9 |
| 2,3-lutidin | 1.20 | 32.0 | 75.5 | 4.0 | 7.7 | 0.6 |
| 3,5-lutidin | 1.12 | 35.4 | 82.8 | 2.0 | 3.6 | 0.4 |
| 3,4-lutidin | 1.10 | 34.4 | 80.2 | -1.2 | -3.6 | 0.4 |
| 2-picoline | 1.18 | 31.9 | 75.0 | -1.4 | -4.6 | 0.6 |
| 3-picoline | 1.08 | 33.1 | 77.0 | -0.6 | -2.0 | 0.3 |
| 4-picoline | 1.08 | 32.0 | 74.6 | 0.7 | 0.9 | 0.3 |
Table 5: Thermodynamic parameters of dissolution of substituted pyridines in the nematic and isotropic phases of LC-2 at infinite dilution.
| Sorbate | γ ∞ | –ΔH∞, kJ/mol | –ΔS∞, J/(mol·K) | HE, kJ/mol | SE, J/(mol·K) | GE, kJ/mol |
|---|---|---|---|---|---|---|
| T=443 K | ||||||
| 2,6-lutidin | 2.29 | 13.1 | 36.4 | 19.0 | 35.9 | 3.0 |
| 2,5-lutidin | 1.50 | 16.3 | 40.2 | 18.7 | 38.8 | 1.5 |
| 2,4-lutidin | 1.54 | 16.6 | 41.0 | 17.4 | 35.6 | 1.6 |
| 2,3-lutidin | 1.47 | 15.7 | 38.8 | 20.1 | 42.1 | 1.4 |
| 3,5-lutidin | 1.24 | 15.7 | 37.4 | 21.5 | 46.6 | 0.8 |
| 3,4-lutidin | 1.14 | 17.6 | 40.7 | 13.5 | 29.4 | 0.5 |
| 2-picoline | 1.41 | 12.7 | 31.7 | 17.4 | 36.3 | 1.3 |
| 3-picoline | 1.16 | 14.6 | 34.2 | 17.6 | 38.4 | 0.6 |
| 4-picoline | 1.05 | 14.9 | 34.2 | 17.4 | 38.8 | 0.2 |
Table 6: Thermodynamic parameters of dissolution of substituted pyridines in the nematic phase of mesogen Ia at infinite dilution.
Peculiarities of thermodynamic compensation effect were analyzed by plotting the dataset in the 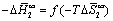 coordinates (Figure 10). The data unequivocally evidenced about prevailing of the entropy factor in dissolution of isomeric pyridine derivatives, due to steric limitations imposed by a liquid-crystalline matrix.
coordinates (Figure 10). The data unequivocally evidenced about prevailing of the entropy factor in dissolution of isomeric pyridine derivatives, due to steric limitations imposed by a liquid-crystalline matrix.
Contribution of the hydrogen bond strength between the liquid crystal and the non-mesogen into the retention selectivity we performed quantum-chemical simulation of mesogens, sorbates, and their complexes (Figure 11). Geometry optimization as well as computation of the force field and the vibrations frequency was performed taking advantage of the DFT method (B3LYP hydrid functional) with the Dunning split-valence basis set cc-pVTZ. The simulation consisted of two stages: first, the starting geometry was optimized using the Hartree- Fock method; second, the configurations corresponding to the minima at the potential energy surface were used as starting ones for DFT analysis. The simulation was performed using PC GAMESS software [63]. Data input preparation and results processing were carried out using Chem Craft software [64].
The simulation demonstrated that the bond energy was almost the same in all the complexes. That confirmed the previously discussed prevailing of the steric factor over the energy one in dissolution of the isomeric lutidins and picolines. Hence, the factor of steric limitations on the sorbate incorporation into the liquid-crystalline stationary phase was crucial to determine efficacy of separation of the electrondonating pyridine derivatives. The prominent selectivity was due to the high orientation ordering of the terminal substituents resulting from either their short length or the supramolecular hydrogen bonding interaction of the complementary groups.
In this work we have presented the studies of structure and physical properties of supramolecular 4-(ω-hydroxyalkyloxy)-4’- cyanoazoxybenzenes. The prepared compounds contain terminal -CN and -OH groups in a single molecule. These groups are highly reactive, allowing for the structure modification to afford synthons for targeted synthesis of novel macroheterocyclic and mesogenic materials. Furthermore, these terminal substituents are capable of specific intermolecular interactions.
We have demonstrated that self-assembly of azoxybenzenes Ia-e containing active functional substituents decreases the tendency of the material to form smectic phase, enhances thermal stability of the nematic phase, and increases the dielectric anisotropy. Doping of liquid-crystalline 4-pentyloxy-4’-cyanobiphenyl with small amount of the prepared supramolecular azoxybenzenes Ia-e has resulted in the increase of dielectric permittivity anisotropy and birefringence. The effect is likely caused by formation of either supramolecules linked via the ~??···??~ hydrogen bonds or linear supramolecular assemblies containing the ~??···NC~ linkage.
We have analyzed sorption and selective properties of the stationary phase based on supramolecular 4-(2-hydroxyethyloxy)- 4’-cyanoazoxybenzene Ia. The mesogen exhibits high structural selectivity with respect to various isomers, unachievable when using “conventional” nematic azoxybenzenes; that is due to self-assembly into the chain associates. The prevailing factor of the high structural selectivity of the supramolecular mesogen is the entropy contribution resulting from the limited mobility of terminal groups after the mesogen self-assembly.
This work was financially supported by Russian Scientific Foundation (Agreement No. 14-23-00204).