Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2017) Volume 8, Issue 4
With this study, we aimed to determine the polyphenol composition and antiradical activity of activity of Erica arborea a native plant species from the Portuguese and Mediterranean flora and often reported as having important bioactivities, but without consistent scientific evidences. The analysis of polyphenol profile and content was performed by HPLC-DAD/UV-Vis and in vitro bioassay of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) method was used to evaluate the radical scavenging activity. The HPLC analysis showed a great diversity of compounds. E. arborea presented high contents in flavonols (52%) and flavanols (25%) and the average content of antiradical activity higher than 85%. The polyphenols identified are often associated by literature as having preventive properties and protective effect against of degenerative and inflammatory processes, thus, our findings confirm the empirical health properties often associated to this endemic plant. Therefore, based in our study the E. arborea, can be used to extract and purify phytochemicals with potentially beneficial effects on health.
Keywords: Endemic flora; Phytochemicals; Bioactivities; Pharmaceutical value
The genus Erica is the largest genus of the family Ericaceae with 67 genera with 1,400 species mainly confined to the Europe and Asia [1]. Erica arborea (white flowers) are member of this family and are largely present in the mountains of Northern Portugal and Spain, but it is possible to find in other areas of Mediterranean basin. Despite this predominance in these regions no systematic studies have been made, at least to our knowledge, about their phytochemical composition and biological potential. Although, it is well known that Ericaceae species are used for centuries in folk medicine to treat urinary tract infections and other inflammatory diseases [2-4], none of their claimed properties were consistently proven. Different studies have shown that Ericaceae plants contain a larger number of biologically active secondary metabolites, including terpenoids, tannins and polyphenols [5-7]. Recently, investigations have shown that Ericaceae plants such as Arbutus unedo and Erica australis, largely present in the same region, are used in folk medicine as infusions and teas to treat diabetes, hypertension, prostate inflammation, bladder, kidneys diseases, and they are claimed to have anti-inflammatory and sedative properties [8-11]. It was also found that E. arborea displayed remarkable anti-inflammatory and antinociceptive activities due to its richness in phytochemicals, particularly phenolic acids, flavonoids and tannins which seems to be responsible for the high antiradical activities observed [12]. Another studies with other Ericaceae plant species, Gaultheria procumbens and Gauhheria leucocarpa, showed that high content of salicylic acid derivatives, especially methyl salicylate, might be responsible for its claimed strong anti-inflammatory and anti-rheumatoid properties [13,14]. Despite the large presence of these shrubs in Portugal and Iberian landscapes, no comprehensive profile of these species has been reported. Moreover, their putative beneficial effects are highly speculative, and no systematic and consistent study have been made to correlate their bioactivities with full phytochemical characterization, particularly polyphenols. In fact, polyphenols have been associated with important beneficial effects in the human health. In the past recent decades, epidemiological studies have shown that polyphenols have several biological benefits, such as reducing the risk of appearance of chronic diseases, scavenging oxidative and toxic free radicals, antiaging and antimicrobial properties. The objective of the present study was to characterize the phenolic composition of E. arborea by HPLC-DAD/ UV-Vis and to investigate their biological value by an in vitro radical scavenging activity. This information will help to clarify if both plants can be used as natural source of phytochemicals by pharmaceutical and agro-food industry.
Plant material and sampling
One kilogram of fresh weight of Erica arborea (leaves and flowers) were collected during the spring season at April 2015 in the natural open fields in Northern Portugal, Vila Real Region (400 meters of altitude), Natural Park of Alvão (N 41°17'35.538", W 7°44' 29.6268"). After harvest, the fresh samples were properly and botanically identified and dried in a freeze-drier system (UltraDrySystemsTM, USA), milled and reduced to a fine powder, and stored in dark flasks at 4 °C in a dark environment until extraction. Fresh and dry weight were registered and the amount of dry matter was determined. Three replications were taken from independent biological samples.
Extraction procedure
One hundred mg of powdered dry samples were placed in a screw cap tubes (10 mL) and mixed with 10 mL of 70% aqueous methanol (methanol:water). Each mixture was vigorously agitated in a vortex (Genie 2, Fisher Scientific, UK), heated at 70 °C (1083, GFL-Gesells chaft ffur Labortechnik mbH, Germany) during 20 minutes and agitated every 5 minutes. Then, the mixtures were centrifuged at 4000 rpm and 15 min (Kubota, Japan), and filtered through a Whatman No. 1 paper. The supernatants were then filtered through PTFE 0.2 μm, Ø 13 mm (Teknokroma, Spain) to amber HPLC vials (Chromabond 2-SVW(A) ST-CPK, Sigma-Aldrich, Tauferkichen, Germany). Finnaly, the obtained extracts were stored (no more than one week) under refrigeration (-20 °C) prior to the HPLC and antiradical colorimetric bioassay analysis.
Polyphenol composition by HPLC-DAD/UV-Vis
The separation and quantification of polyphenols present in E. cinerea and E. arborea hydro-alcoholic extracts were performed by HPLC-DAD-UV/VIS [15]. After extraction, each extract was injected in HPLC-DAD/VIS-UV system (Gilson Inc., Middleton, WI, USA) equipped with an eluent composed by water with 1% of trifluoroacetic acid (TFA) (solvent A) and acetonitrile with 1% TFA (solvent B). The elution was performed at a flow rate of solvent of 1 mL min-1, with a gradient starting with 100% of water, with an injection volume of 10 μL. The chromatograms were recorded at 280, 320, 370 and 520 nm with a C18 column (250 × 46 mm, 5 μm) (ACE® HPLC columns, Advanced Chromatography Technologies, Ltd., Aberdeen, Scotland). The polyphenols were identified using peak retention time, UV spectra and UV max absorbance bands and trough comparison with external commercial standards (Extrasynthese, Cedex, France, and Sigma-Aldrich, Tauferkichen, Germany), as well as by comparing with published literature [16-19] The external standards were freshly prepared in 70% methanol (methanol:water) at concentration of 1.0 mg mL-1 and running in HPLC-DAD-UV/VIS before the samples. The quantification was done using response factor for each compound detected compared to each similar pure standard compound. The results were expressed as μg g-1 dry weight (dw). Methanol and acetonitrile were HPLC gradient and purchased from Panreac chemistry (Lisbon, Portugal) and Sigma-Aldrich (Taufkirchen, Germany), respectively. The aqueous solution were prepared using ultra-pure water (Milli-Q, Millipore, Massachusetts, USA).
ABTS radical scavenging activity
The ABTS radical scavenging activity of E. arborea extracts was evaluated using the (ABTS●+) radical-scavenging activity colorimetric method [20] conducted in a 96-well microplate. For that different concentrations of both hydro-alcoholic extracts were prepared, ranging from 0.195 to 10.0 mg mL-1 and used in the in vitro AA assay. A radical ABTS solution was freshly prepared by mixing a 7 mM of ABTS at pH 7.4 (5 mM NaH2PO4, 5 mM Na2HPO4 and 154 mM NaCl) with 2.5 mM potassium persulfate (final concentration) followed by storage in the dark at room temperature for 16 h before use. After, the mixture was diluted with ethanol to give an absorbance of 0.70 ± 0.02 units at 734 nm using a multiscan microplate reader (Multiskan™ FC Microplate Photometer, USA). Then, to each well, an aliquot of 15 μL of each extract or standard were added followed by addition of 285 μL fresh ABTS solution. The microplates were then incubated at room temperature and dark during 10 minutes. At the end, the absorbance values were measured in a multiscan microplate reader at 734 nm. The results were expressed as percentage (%) of ABTS radical scavenging activity, using the following formula:
ABTS radical scavenging (%)=[((absorbance solvent‐absorbance sample)/absorbance solvent) × 100]
The concentration of antioxidants which scavenge the free radical ABTS• about 50% (IC50) for both plant extracts was also determined. A positive control with high recognized antioxidant and antiradical activity, the ascorbic acid, was included in the AA assay.
Statistical analysis
All determinations were carried out in triplicate and the results were expressed as mean values ± standard deviation (SD). The Software SPSS v.17 (SPSS-IBM, Orchard Road-Armonk, New York, USA) was used to carry out these analysis.
The analysis by HPLC-DAD/UV-Vis of the methanolic extracts of E. arborea revealed the presence of different classes of polyphenols as illustrated in the Figure 1, in which is presented a typical example of chromatogram find in the current study. The quantity of each polyphenol identified in each plant extract is presented in Table 1, in which is also included the data of retention time and λ max in the visible region for each polyphenol, and in Table 2 is presented the chemical structure of each polyphenol identified. According our results, the major individual polyphenols were quercetin-3-O-rutinoside (13% of total polyphenols identified), 5-O-p-coumaroylquinic acid (12%), kaempferol-3-Orutinoside (12%), epicatechin (11%) and myricetin-3-O-rhamnoside (8%), which are reported by literature as having important bioactive properties, such antiradical and antioxidative capacity. However, our results showed a lower antiradical activity when compared to a positive control with recognized antioxidant or antiradical activity, the ascorbic acid (Figures 2 and 3), but when compared to other medicinal plants [21,22] the E. arborea showed a moderate to higher antiradical activity.
| Polyphenols | Rt (min) | UV detection (nm) | UV λmax (nm) |
Quantity (µg g-1 dw) |
|---|---|---|---|---|
| trans-cinnamic acid | 15.72 | 320 | 317 | 3.5±0.2 |
| 5-O-caffeoylquinic acid | 16.45 | 320 | 280, 320 | 8.6±0.9 |
| Gallocatechin | 17.36 | 280 | 271 | 25.5±0.9 |
| Myricetin-3-O-glucoside | 18.21 | 370 | 271, 347 | 56.8±1.0 |
| Myricetin-3-O-galactoside | 18.32 | 370 | 276, 346 | 20.4±0.8 |
| Epigallocatechin | 18.78 | 280 | 276 | 15.2±0.2 |
| Myricetin-3-O-rhamnoside | 19.16 | 370 | 276, 342 | 64.0±1.1 |
| Epicatechin | 19.67 | 280 | 277 | 91.0±0.4 |
| Epigallocatechin gallate | 19.93 | 280 | 279 | 38.3±0.6 |
| p-coumaroylquinic acid | 20.02 | 320 | 282, 311 | 11.9±1.0 |
| Gallocatechin gallate | 20.38 | 280 | 277 | 15.7±2.3 |
| Kaempferol-3-O-rutinoside | 20.67 | 370 | 265, 358 | 97.5±2.7 |
| Kaempferol-3-O-galactoside | 20.79 | 370 | 265, 356 | 49.4±1.6 |
| Myricetin | 21.03 | 370 | 275, 341 | 5.8±0.2 |
| Kaempferol-3-O-glucoside | 21.44 | 370 | 267, 355 | 11.2±0.7 |
| Epicatechin gallate | 22.71 | 280 | 278 | 23.1±0.5 |
| Quercetin-3-O-rutinoside | 22.08 | 370 | 253, 354 | 109.7±0.2 |
| Quercetin-3-O-galactoside | 22.21 | 370 | 256, 355 | 8.7±1.9 |
| 5-O-p-coumaroylquinic acid | 22.30 | 320 | 285, 312 | 99.1±0.1 |
| Ellagic acid | 24.56 | 370 | 354 | 56.4±0.2 |
| Chrysin | 26.04 | 370 | 256, 313 | 11.3±1.0 |
| Kaempferol | 26. 39 | 370 | 266, 357 | 4.1±0.4 |
Values expressed as mean ± standard deviation (SD) of three replicates
Table 1: Profile and contents of polyphenols, respective retention time (Rt), maximum absorption (λ max) in methanolic extracts of Erica arborea (by elution order).
| Polyphenols and chemical structures | ||
|---|---|---|
5-O-caffeoylquinic acid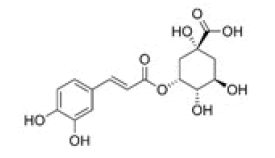 |
Gallocatechin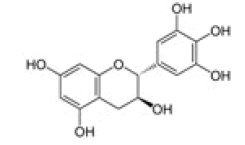 |
Kaempferol-3-O-rutinoside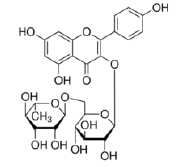 |
5-O-p-coumaroylquinic acid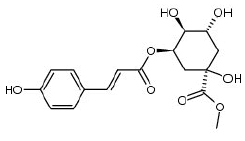 |
Gallocatechin gallate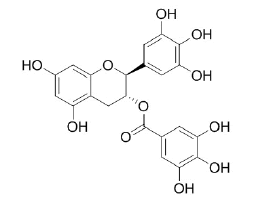 |
Myricetin-3-O-galactoside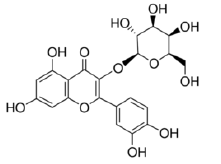 |
Chrysin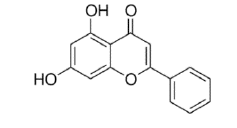 |
Epigallocatechin gallate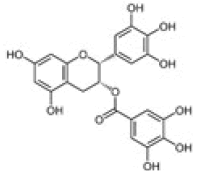 |
Myricetin-3-O-glucoside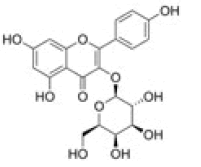 |
Ellagic acid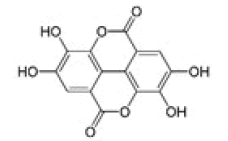 |
Kaempferol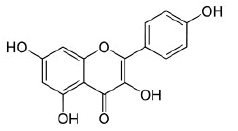 |
Myricetin-3-O-rhamnoside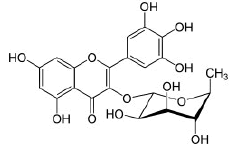 |
Epicatechin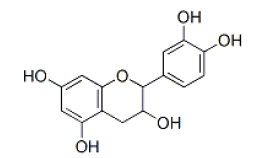 |
Kaempferol-3-O-galactoside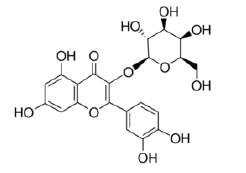 |
Quercetin-3-O-galactoside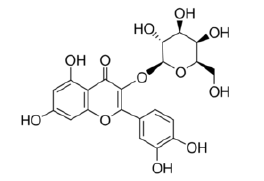 |
Epicatechin gallate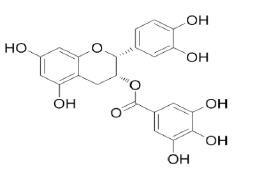 |
Kaempferol-3-O-glucoside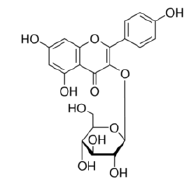 |
Quercetin-3-O-rutinoside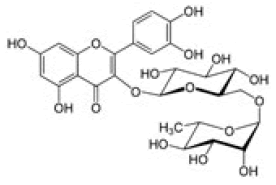 |
Table 2: Chemical structures of the main polyphenols identified Erica arborea extracts, by alphabetical order.
Polyphenols, antioxidant and antiradical activities have been considered parameters to measure the biological property of foods. In our study, extracts of E. arborea revealed the presence of different classes of polyphenols, often associated with anti-inflammatory activities in other plant matrices. Our results also shown that flavonoids (kaempferol, myricetin, quercetin and luteolin glycosides) and phenolic acids (chlorogenic, neochlorogenic and caffeic acids) are associated with the antiradical activity. These compounds act as scavengers of various oxidizing species such as superoxide anion, hydroxyl radical or peroxy radicals and as quenchers of singlet oxygen [23] and their antiradical capacity arise from their ability to inhibit lipid peroxidation, chelate redox-active metals, and reduce all processes involving reactive oxygen species [24]. They may exert protection of cell structures through different mechanisms, and one in particular is their ability to increase the levels of the powerful intracellular antioxidant enzymes [25,26]. Also, recent works have demonstrate that phenolic acids like chlorogenic and neochlorogenic acids can have a strong anti-inflammatory activity [27,28]. Similar observations were made recently to caffeic acid by Song et al. [27] who reported that caffeic acid is able to inhibit the activity of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) in a dose-dependent manner, protecting the brain against neuronal inflammatory processes and diseases. Therefore, the high content of such important compounds in E. arborea may be used as an argument to explain their high biological potential, and may enhance the interest to study this plant species as natural source to extract and purify bioactive compounds for pharmaceutical and agro-food industry.
The authors acknowledge the support of European Investment Funds by FEDER/COMPETE/POCI–Operational Competitiveness and Internationalization Programme, under Project POCI-01-0145-FEDER-006958 and National Funds by FCT, Portuguese Foundation for Science and Technology, under the project UID/ AGR/04033/2013.
The authors declare no conflict of interest.