
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)Volume 10, Issue 5
Background: Since the late 2019, SARS-CoV2 has spread worldwide, leading WHO to declare a pandemic state. Italy resulted deeply affected by the infection, especially the North. Although there is no proven specific treatment for the infection and for the pulmonary disease, several molecules have been tested always comparing efficacy and collateral effects. Oxygen support plays a key role in the management of this type of patients along with the collaboration with intensive care specialists. This case regards a COVID-19 patient whose clinical parameters deteriorated few days after admission and who had been treated with both Tocilizumab and High Flow Nasal Oxygenation. Case presentation: A 55 yo caucasian female was admitted to ED because of fever and dyspnea. Her chest X-ray showed bilateral ground glass areas and blood examinations revealed elevated inflammatory markers along with lymphopenia. RT-PCR for SARS-CoV2 resulted positive and the patient was transferred to our Unit. We started administration of antiviral therapy with Lopinavir/ritonavir, hydroxychloroquine plus azithromycin, along with antibiotic therapy and anticoagulant treatment. Despite the therapy, her clinical conditions started deteriorating together with chest X-ray findings and arterial blood gas parameters. Inflammatory markers were elevated too. We started High Flow Nasal Cannula Oxygenation (HFNC) along with Tocilizumab administration. In two days, respiratory frequency and arterial blood gas data were ameliorated and chest X-ray improved too. In six days, she started waning HFNC and, after ten days, she stopped. Due to the important clinical and radiological amelioration along with two negative results on RT-PCR for SARSCoV2, the patient was discharged. Conclusions: It is indisputable that we need more data and guidelines about COVID19 therapies that lead us through the correct clinical behavior to guarantee the best treatment for these patients
COVID-19 pandemic; COVID-19 virus disease; COVID-19 treatment
SARS-CoV2: Severe Acute Respiratory Syndrome Coronavirus; COVID-19: Coronavirus Disease-19; ARDS: Acute Respiratory Distress Syndrome; Hb: Hemoglobin; WBC: White Blood Cells; ESR: Eritrosedimentation Rate; CRP: C Reactive Protein; LDH: Lactic Dehydrogenase; RR: Respiratory Rate; HFNC: High Flow Nasal Cannula; IDSA: Infectious Diseases Society of America; SIMIT: Italian Society of Infectious Diseases; HBV: Hepatitis B Virus; IFN: Interferon; NIV: Non-Invasive Ventilation; RCT: Randomized Controlled Trial
In late 2019, pneumonia of unknown origin was reported in Wuhan, China [1]. The causative pathogen has been soon identified as a novel beta-coronavirus, subsequently named Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV2) [1,2]. Since then, there has been a rapid spread of the virus worldwide, leading the World Health Organization to declare SARS-CoV2 outbreak a global pandemic on March 11, 2020 [3].
Most infected individuals who develop disease from SARSCoV2 infection (COVID-19) exhibit a mild to moderate illness (80%); however, 14% have serious disease and in 5% it evolves in a severe Acute Respiratory Distress Syndrome (ARDS) requiring intensive care support [4].
Up to the 8th of June 2020, more than 7 million cases of SARSCoV2 infections have been reported and 406.353 people died worldwide. So far, a total of 234.998 cases have been reported in Italy, 33.899 of which have died [5].
Two main phases of the disease have been recognized, a “viral phase” with mild to moderate symptoms and an “inflammatory phase”, which could induce the development of ARDS.
Due to the urgency to find effective therapeutical strategies, many treatments have been investigated to identify drugs both efficacious and safe against COVID-19 main phases [6].
Until now, there are no proven specific antiviral agents to treat COVID-19; however, several new and old molecules have been used in the context of clinical trials while waiting for solid evidences to make drugs administration safer and more precise [6].
As regards the second phase, one of the most studied treatments is the monoclonal antibody Tocilizumab that blocks the cellular receptor of IL-6, which plays a crucial role in the development and maintenance of inflammation.
Oxygen support along with Intensive care specialists’ assistance play a pivotal role in the management of severe COVID-19 cases [7]. In that context, High Flow Nasal Oxygenation represents an essential support in the governance of these critical patients.
Here we described a successful clinical case of a patient with COVID-19 that became difficult to deal with in few days after admission.
A 55 years old Caucasian female was admitted to ED after 5 days of high fever (peak at 39°C), along with cough and shortness of breath. Chest X-ray showed bilateral ground glass areas and blood tests revealed elevated serum inflammatory markers as well as lymphopenia. Her medical history was unremarkable. She did not take any drugs.
On March 21st, due to a positive result of SARS-CoV2 RT PCR on nasopharyngeal swab, the patient was transferred to our unit.
On admission, she was febrile (T: 38.5°C), blood pressure was 140/80 mmHg, heart rate 100 bpm, oxygen saturation 91% in room air, respiratory rate (RR) was 24/min.
Blood tests showed mild anemia (Hb 11 g/dl), normal WBC count (7500 cell/mmc) with lymphopenia (Neutrophils 77.6%, Lymphocytes 14%), high inflammatory markers (ESR 45 mm/h, CRP 2.42 mg/dl, IL-6 1500 pg/ml, D-Dimer 1200 ng/ml, LDH 280 U/L), irrelevant procalcitonin levels. Pneumococcal and Legionella urinary antigens were negative.
Arterial blood analysis in room air showed a paO2 50 mmHg, pCO2 35 mmHg, and pH 7.44; P/F ratio was 238.
Her chest X-ray showed bilateral ground glass areas without consolidations (Figure 1). Her ECG was normal (QTc was 436).
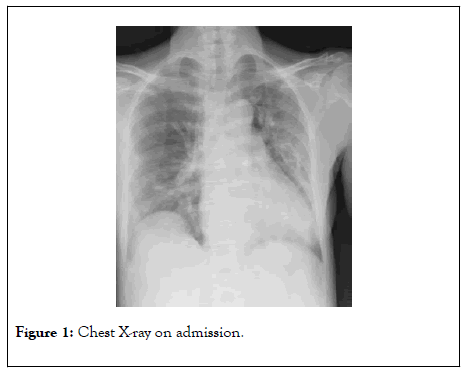
Figure 1: Chest X-ray on admission.
She started Lopinavir/ritonavir (200 mg/50 mg 4 tabs/day per os, for 5 days), hydroxychloroquine 400 mg/die per os (for 7 days, after loading dose), azithromycin 500 mg/die per os (for 7 days), ceftriaxone 2 gr/die iv (for 7 days), heparin 6000 UI/die subcutaneous, as per protocol.
Furthermore, she started oxygen support therapy with Venturi mask (12 lt/min, FiO2 60%).
On March 23rd, despite the therapy, her clinical conditions started deteriorating, with chest X-ray worsening (Figure 2). She became dyspneic (RR was 30/min) and arterial blood analysis showed paO2 80 mmHg, pCO2 33.4 mmHg, pH 7.38 (P/F ratio was 133).
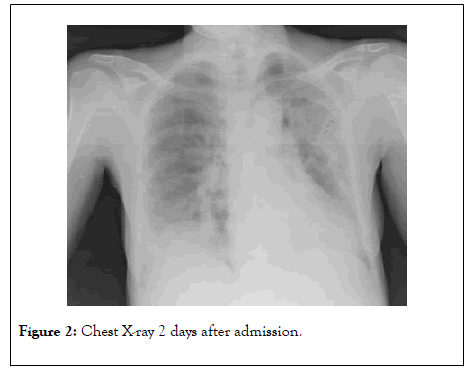
Figure 2: Chest X-ray 2 days after admission.
According to intensive care specialists, she started HFNC (OptiflowTM Nasal High Flow Therapy delivered by AIRVOTM 2) ventilation (50 lt/min, FiO2 60%) and, due to elevated serum IL-6 levels and other serum inflammatory markers levels (such as DDimer), Tocilizumab had been administered intravenous, two 8 mg/kg doses 12 hours apart.
On March 25th, her clinical conditions were dramatically ameliorated, arterial blood analysis on HFNC oxygenation, showed paO2 86 mmHg, pCO2 34.6 mmHg, pH 7.37 and P/F ratio was 172. Chest X-ray also improved (Figure 3). Serum inflammatory markers were also decreased (IL-6 400 pg/ml, DDimer 540 ng/ml, CRP 1.2 mg/dl).
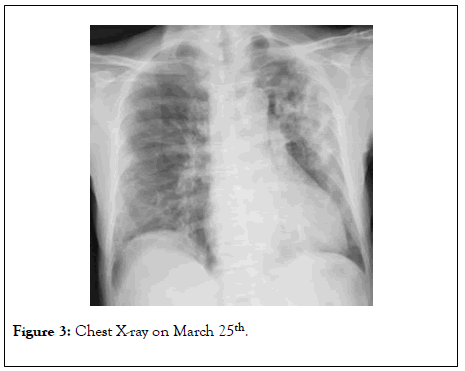
Figure 3: Chest X-ray on March 25th.
After six days, she started waning HFNC and interchanging it with Venturi mask every six hours until April 3rd, when she stopped HFNC and continued only with Venturi mask for other three days; on that date, RT-PCR for SARS-CoV2 on nasopharyngeal swab resulted negative.
On April 6th, arterial blood analysis in room air showed paO2 86 mmHg, pCO2 36 mmHg, pH 7.33 with P/F ratio of 409. Chest X-ray was markedly ameliorated (Figure 4). Blood tests showed normalization of inflammatory markers along with resolution of lymphopenia. Moreover, a second RT-PCR on nasopharyngeal swab was negative for SARS-CoV2.
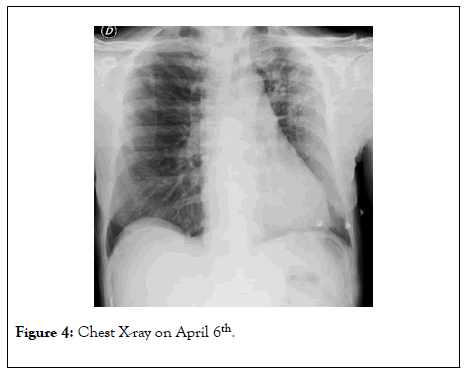
Figure 4: Chest X-ray on April 6th.
On April 10th, the patient was discharged and sent home, eupneic, afebrile and in good clinical conditions.
Up until now, a lot of literature has been made, especially about pharmacological therapy against SARS-CoV2 infection, most of which showing unclear results or undefined clinical benefits. We relied on IDSA guidelines [6] and Italian vademecum (SIMIT) [8] updated up to the moment in which the patient was admitted.
It has been shown that there are two main pathogenetic moments in the development of COVID-19. A “viral phase” due to viral intracellular replication, made up of mild symptoms such as fever, dry cough, diarrhea, and headache [9]; and an “inflammatory phase” due to the host immune response, made up of severe respiratory symptoms up to ARDS along with serum inflammatory markers huge increase, the so called “ cytokine storm ” . In each of these phases, we are able to intervene with different weapons.
Up until now, the therapy for the viral phase, has been based on the antiviral activity, either real or supposed, of some molecules such as Lopinavir/ritonavir [10,11], hydroxychloroquine [12,13] and azithromycin [14] along with heparin, in prophylactic or therapeutic dosage, to treat the ipercoagulation state (up to Disseminated Intravascular Coagulation) due to the high inflammation status [15,16].
However, due to lack of evidence about the efficacy and the benefits of these treatments, IDSA guidelines [6] recommend the use of antiviral drugs only in the context of clinical trial, with accurate caution to the collateral effects, which may be particularly pernicious (such as QT prolongation for azithromycin and hydroxychloroquine as well as important bleeding for heparin or diarrhea with Lopinavir/ritonavir).
According to the patient’s clinical conditions, we decided to administer a short course of antiviral treatment and prophylactic dosage of heparin. She had no adverse drug reactions. Moreover, we assessed QTc daily without evidence of prolongation.
SARS-CoV-2 binds to alveolar epithelial cells and it considerably triggers innate and adaptive immune system with consequent massive release of a large number of cytokines, initializing the “cytokine storm”, which plays a central role in the development of severe COVID-19 [17].
Due to the role of these pro-inflammatory molecules, vascular permeability increased, a substantial amount of fluid and blood cells come into the alveoli, resulting in an interstitial pneumonia that leads to deep pulmonary damage which highly reduces respiratory capacity [14,17].
Patients suffering from cytokine storm, occurring due to huge level of immune activation of macrophages, lymphocytes and myeloid cells [18], progress to multiple organ dysfunction with life-threatening complications including fluid-refractory hypotension along with cardiac dysfunction, respiratory failure, coagulopathy, renal and liver failure [19]. Early identification, treatment and prevention of the cytokine storms are of crucial importance for the patients.
IL-6, which is a multieffective cytokine with both antiinflammatory and pro-inflammatory pathways [17], is one of the most important cytokines involved in COVID-19-induced cytokine storms [19], it is a relevant marker of inflammation and can handle the clinicians in recognizing patients with severe COVID-19 early in the disease course [20].
Tocilizumab, which is a recombinant monoclonal antibody targeting IL-6 receptor, seems to be greatly active in the COVID-19 inflammatory phase [6]. It is already used in rheumatoid arthritis, juvenile idiopathic arthritis and Crohn’s disease [17]. Up until now, only small groups of patients or simple studies reported the use of Tocilizumab in severe COVID-19 patients, achieving promising clinical results [17,19].
Tocilizumab can be used in patients with extensive bilateral lung involvements or in severe or critical patients, who have elevated laboratory detected IL-6 levels. The dose is 4-8 mg/kg iv, diluted in 100 ml of 0.9% saline solution. For patient with poor clinical response, a second dose could be administered after 8-12 hours [21].
We used Tocilizumab following strict inclusion and exclusion criteria (Table 1). We administered two 8 mg/kg Tocilizumab doses iv, 12 hours apart. The patient had no infusion reactions nor adverse drug reactions. Prior Tocilizumab administration, we assessed patient HBV status, which was negative, and we performed an IFN-γ essay test to exclude latent Tuberculosis infection that resulted negative too.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Confirmed SARS-CoV2 infection | Age<18 yo |
| Interstitial lung involvement confirmed with imaging | Elevated transaminase levels (5 x) |
| Oxygen saturation<93% in room air or paO2/FiO2 ratio<300 | Confirmed bacterial infections or sepsis |
| High serum levels of IL-6 (>40 pg/ml) | Diverticulitis or gastrointestinal perforation |
| Other immunosuppressive treatment |
Table 1: Inclusion and exclusion criteria for Tocilizumab administration.
High flow oxygen systems (such as High Flow Nasal Cannula, HFNC) supply heated, oxygen-improved, humidified gas to the patient at flow levels adequate to release a constant and exactly set high FiO2 [7]. HFNC flow rates reach up to 60 L/min. HFNC reduces pulmonary dead space, provides low levels of PEEP, and decrease breathing frequency and breathing effort [22,23]; its use is associated with lower mortality in hypoxemic respiratory failure [24].
Compared to NIV oxygen therapy, HFNC is associated with decreased need of subsequent intubation and ICU admission [25-27]. HFNC was associated with lower risk of 30 days mortality in patients with pneumonia and patients without hypercapnia compared to NIV [28]. Moreover, HFNC are more comfortable and so more tolerated than NIV and the management of HFNC is much easier than NIV [29].
Furthermore, HFNC show a significant low risk of bioaerosol dispersion reducing the risk of hospital-acquired infection for health workers [22]. In a retrospective study of 610 patients from China, where 10% of the affected patients needed critical care, an aggressive and early use of HFNC was associated with reduced necessity of mechanical ventilation and lower mortality [30,31].
After our patient started HFNC ventilation, she achieved a remarkable improvement in respiratory function as well as lower respiratory fatigue, with ameliorated results on arterial gas analysis. We started with HFNC all day long, to then interchanging it with Venturi mask (every six hours) to avoid sudden interruption.
Frequent chest X-rays along with arterial gas analysis, as well as the strict collaboration with intensive care specialists, helped us manage this successful case.
SARS-CoV2 infection arrived “out of the blue” for the entire world, even in Italy.
In Sicily, south Italy, we have been warned by what had already happened in North Italy. Moreover, we have been helped by lockdown measures and by other countries therapeutical experiences.
In our experience, COVID-19 patients progressing towards a more severe course might benefit of intravenous Tocilizumab; nevertheless, we claim as critical to define the correct timing in the drug administration. Also, oxygen ventilation with HFNC could be a synergic support with a pivotal role in the amelioration and resolution of the disease.
We need more data about these therapies from solid RCT and guidelines that lead us through the correct clinical behavior. Meanwhile, we hope this case will help manage other similar patients in the same clinical conditions.
Ethics approval and consent to participate.
Consent to publish.
Written informed consent for publication of clinical details was obtained from the patient and it is contained in the patient’s clinical record.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. We used only information contained in the patient’s clinical record.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AM and FC wrote the paper. AP, DS, VM, MC revised literature and references.
MG, AO, AZ, ST, SG, BMC, FB, VB, GV, LL and SB gave clinical assistance to the case. RB handle the lab tests and pharmacological treatment. GN and BC revised the paper.
All authors read and approved the final manuscript.
We thank Dr. PietroLeanza for his kind English revision.
Citation: Marino A, Cosentino F, Pampaloni A, Scuderi D, Moscatt V, Ceccarelli M, et al. (2020) Role of Tocilizumab and High Flow Nasal Cannula In the Clinical Management of Severe Covid-19. J Clin Trials 10:427. doi: 10.35248/2167-0870.20.10.427
Received: 17-Jul-2020 Accepted: 30-Jul-2020 Published: 06-Aug-2020
Copyright: © 2020 Marino A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.