Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Review Article - (2023)Volume 11, Issue 3
Worldwide, Acute Diarrhoea (AD) is a very common illness that significantly increases morbidity and death. Oral Rehydration Solutions (ORS), antibiotics and zinc products are examples of conventional treatments. New research indicates that probiotics may be used to enhance current medications for additional control in the treatment and management of AD cases in youngsters.
Here, we examine the research supporting several probiotic treatment approaches for AD. We cover the prevalence, clinical impact and complications of acute diarrhoea in children before giving a general summary of available therapies and concluding with talking about recent emergent gut approaches to AD care. We will present in a comparative research-the effectiveness of various types of probiotics known and utilised, as well as typical natural items, in the treatment of acute instances of AD and concentrate on recent, high-quality studies. Adverse effects and potential interactions of each therapy will be highlighted where applicable.
Acute diarrhea; Probiotic supplements; Gut microbiota; ORS; Natural agents
Probiotics are live microorganisms that are intended to have health benefits when consumed or applied to the body. They can be found in yogurt and other fermented foods, dietary supplements and beauty products [1]. Probiotic products have special microorganisms like bacteria or yeast in them. These are thought to reach the bowel, where they suppress the germs that are causing the diarrhea and help the body fight them. The best known probiotics are lactic acid bacteria (Lactobacilli) [2].
Probiotics might cut bouts of infectious diarrhea by half a day to about 2 days. Some research shows that the bacteria strains most likely to help are Lactobacillus reuteri, Lactobacillus rhamnosus, and the probiotic yeast Saccharomyces boulardii, although other strains might be useful.
The idea of a healthy gut barrier and a normal, balanced microbiota is the foundation of the contemporary understanding of probiotic therapy. The gut barrier was initially thought of solely as a physical barrier to the admission of foreign antigens until its active function in directing the immune response to luminal antigens was understood. As a result, the scientific notion of these host defences has altered. In addition, new experimental and clinical investigations show that the ability to overcome antigen challenges depends on a healthy host-microbe interaction and as a result, they link the gut microbiota to an active involvement in barrier function [3].
Treatment of acute infectious diarrhoea is the probiotic intervention with the most extensive body of research. Probiotics including Lactobacillus rhamnosus GG, L. reuteri, L. casei shirota and Bifidobacterium lactis Bb12 have been found in wellcontrolled clinical research to shorten [4].
The potential mechanisms by which probiotics fight infectious diarrhoea include exclusion of pathogens by means of competition for binding sites and available substrates, lowering of luminal pH and production of bacteriocins and promotion of the production of mucus-that is, commensal pathogen cross talk. The capacity of pathogenic bacteria to produce mucinase may indeed partly explain the superiority of probiotic therapy in viral diarrhoea. Enhancement of intestinal motility and upregulation of genes mediating innate immunity by probiotics further contribute to the eradication of pathogens [5]. Lactobacilli and bifidobacteria produce short chain fatty acids which regulate cell growth and differentiation and have trophic effects on the intestinal epithelium and additionally stimulate bifidobacteria and counteract urease producing strains, thus maintaining gut barrier function. A Lactobacillus strain isolated from human microbiota was recently shown to counter cellular damage associated with a diarrhoeagenic pathogen, thus corroborating reports of a normalising of intestinal permeability by selected probiotics. Furthermore, specific probiotic strains balance the generation of pro- and antiinflammatory cytokines, thereby creating the healthy hostmicrobe cross talk needed to keep inflammatory responses in check but concomitantly readily primed. Sustained inflammatory activation is required for immunological surveillance at the major portal of entry of pathogens, while the anti-inflammatory effect of specific strains of gut microbiota is requisite to prevent the evolution of infectious diarrhoea toward a protracted course or other chronic sequelae such as allergic and autoimmune disease [6].
Emerging trend in gut management, probiotics in diarrhea country wise prevalence
India leads the chart in child deaths due to diarrhea. More than 386000 children die in India due to diarrhoea every year [7] (Table 1).
Treatment package (UNICEF)
• ORS
• Heralded as one of the most important medical advances of the 20 century
• Cornerstone of fluid replacement
• Low-osmolarity oral rehydration salts
• Zinc treatment-decreases diarrhea severity and duration
• Continued feeding, including breastfeeding, during diarrheal episodes
• Antibiotics
| Rank | Country | Total number of annual child deaths due to diarrhoea |
|---|---|---|
| 1 | India | 386600 |
| 2 | Nigeria | 151700 |
| 3 | Democratic republic of the Congo | 89900 |
| 4 | Afghanistan | 82100 |
| 5 | Ethiopia | 73700 |
| 6 | Pakistan | 53300 |
| 7 | Bangladesh | 50800 |
| 8 | China | 40000 |
| 9 | Uganda | 29300 |
| 10 | Kenya | 27400 |
| 11 | Niger | 26400 |
| 12 | Burkina Faso | 24300 |
| 13 | United republic of Tanzania | 23900 |
| 14 | Mali | 20900 |
| 15 | Angola | 19700 |
Table 1: Children deaths due to diarrhoea occur in just 5 min.
Status of diarrheal disease
An international commitment to tackle childhood diarrhoea in the 1970’s and 1980’s resulted in a major reduction in child deaths. This came about largely through the scaling up of oral rehydration therapy, coupled with programmes to educate caregivers on its appropriate use. But these efforts lost momentum as the world turned its attention to other global emergencies. Today, only 39 per cent of children with diarrhoea in developing countries receive the recommended treatment and limited trend data suggest that there has been little progress since 2000 [8].
Treatment recommendation for pediatric diarrhea
Probiotics may be an effective adjunct to the management of diarrhea. The guidelines recommend the use of the specific probiotic strains, namely Lactobacillus rhamnosus GG (LGG) and Saccharomyces boulardii, for the management of children with Acute Gastroenteritis (AGE) as an adjunct to rehydration therapy [9].
Probiotics basics
Efficacy of any probiotic is strain-dependent. eg:
• Lactobacillus acidophilus strain is effective in IBS but not in AAD.
• Bifidobacterium bifidum reported that one strain (CIDCA 5310) inhibited enterocyte invasion by Salmonella arizonae, whereas the other (CIDCA 537) had no effect (Table 2 and Figure 1).
| Lactobacillus species | Bifidobacterium species | Streptococcus species | Saccharomyces species | Others |
|---|---|---|---|---|
| L. rhamnosus GG | B. bifidum | S. thermophilus | S. boulardii | Bacillus cereus |
| L. casei (rhamnosus) | B. breve | S. salivarius subsp. thermophilus | - | Escherichia coli |
| L. fermentum | B. lactis | - | - | Enterococcus |
| L. gasseri | B. longum | - | - | Propionibacterium freudenreichii |
| L. johnsonii | B. infantis | - | - | |
| L. lactis | B. adolescentis | - | - | - |
| L. paracasei | - | - | - | - |
| L. plantarum | - | - | - | - |
| L. reuteri | - | - | - | - |
| L. salivarius | - | - | - | - |
| L. bulgaricus | - | - | - | - |
Table 2: Common micro-organisms species which are used as probiotics.
Figure 1: Lactobacillus rhamnosus.
Do probiotics work in acute infectious diarrhea?
63 trial, 8014 subjects, results:
• Reduced the duration of diarrhoea by around 25 hours
• Risk of diarrhoea>4 days by 59%
• One fewer diarrhoeal stool on day 2 after the intervention
Study design
To compare the efficacy of 5 different preparations recommended to parents in the treatment of acute diarrhoea in children.
Design: Prospective randomized controlled clinical trial.
Study arm: N=571 children (age 3-36 months).
Duration: 5 days.
Around 525000 children per year die from diarrheal illness, which is the second most common cause of death in children under five. The body may go several days without the salts and water it needs to survive if diarrhoea persists. Past deaths from diarrhoea were typically brought on by acute dehydration and fluid loss [10]. Now, it's likely that a growing number of deaths related to diarrhoea are being caused by other factors, notably septic bacterial infections. The population most at risk for developing life-threatening diarrhoea is children, especially those who are underweight or have weakened immune systems (Table 3) [11].
| Group | Treatment | Dosage (twice daily) |
|---|---|---|
| 1 | Control: Oral Rehydration Solution (ORS) (N=92) | None |
| 2 | Saccharomyces boulardii (N=91) | - |
| 3 | Bacillus clausii (N=100) | 5 billion CFU/dose |
| 4 | Enterococcus faecium (N=91) | 0.075 CFU/dose |
| 5 | Lactobacillus rhamnosus GG (ATCC 53103) (N=100) (uncoated) | 6 billion CFU/dose |
Table 3: Study arms and treatment.
Reduction in duration of diarrhoea
The passage of three or more liquid or loose stools each day (or more frequently) is referred to as diarrhea (Figure 2).
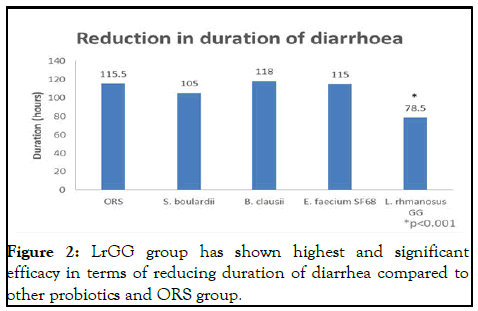
Figure 2: LrGG group has shown highest and significant efficacy in terms of reducing duration of diarrhea compared to other probiotics and ORS group.
Diarrhoea is typically a sign of an intestinal infection, which can be brought on by a number of different bacterial, viral and parasitic organisms [12].
Reduction in daily stool output
Poor hygiene can cause an infection to spread from person to person or through tainted food or drinking water (Figure 3).
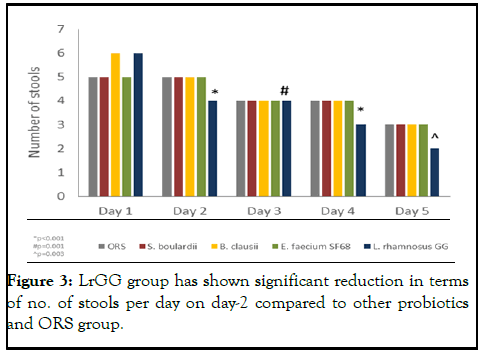
Figure 3: LrGG group has shown significant reduction in terms of no. of stools per day on day-2 compared to other probiotics and ORS group.
Improvement in stool consistency
Diarrhoea prevention measures including using safe drinking water, using better sanitation practises and washing your hands with soap can lower the risk of contracting a disease (Figure 4) [13].
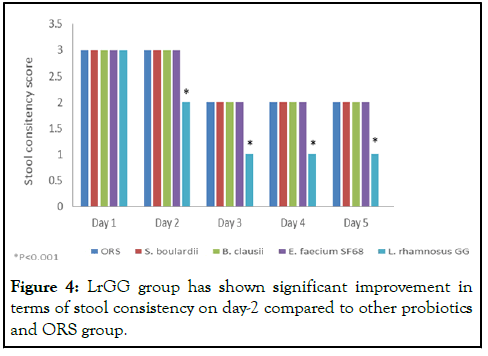
Figure 4: LrGG group has shown significant improvement in terms of stool consistency on day-2 compared to other probiotics and ORS group.
Lactobacillus rhamnosus GG (ATCC 53103)
World’s no.1 researched probiotic:
• Acute diarrhoea
• Persistent diarrhoea
• Rotavirus diarrhoea
• Gastroenteritis
• AAD (prevention and treatment)
• Traveller’s diarrhoea
• Nosocomial diarrhoea
• Irritable Bowel Syndrome (IBS)
• Abdominal pain, crying etc.
• Ulcerative colitis
• Necrotizing Enterocolitis (NEC) in pre-term infants
• As an adjuvant to vaccines to stimulate immunity
• Gastrointestinal infections
• Respiratory infections
L. rhamnosus GG-age group and dosage
Can even be given to a 24 hrs old neonate? Clinically studied in various age group population starting from new born preterm infants to elderly population. Clinically studied at various dosage range starting from 120 mn CFU to 2000 bn CFU per day [14].
L. rhamnosus GG-global recommendations
Oral Rehydration Solution (ORS), a mixture of pure water, sugar and salt, should be used to treat diarrhoea. Additionally, a 10-14 day course of dispersible 20 mg zinc tablets used in addition to standard care reduces the length of diarrhoea and improves results (Table 4) [15].
| S. no. | Authority | Recommendation |
|---|---|---|
| 1 | Nelson textbook of paediatrics | Antibiotic associated diarrhoea |
| 2 | American academy of paediatrics | Acute infectious diarrhoea antibiotic associated diarrhoea |
| 3 | European paediatric association | Nosocomial diarrhoea antibiotic associated diarrhoea acute gastroenteritis |
| 4 | Canadian paediatric society | Anti-biotic associated diarrhoea acute infectious diarrhoea irritable bowel syndrome preventing infections allergic reactions |
| 5 | European society for paediatric gastroenterology, hepatology and nutrition | Acute gastroenteritis |
| 6 | North America society for paediatric gastroenterology, hepatology and nutrition | Antibiotic associated diarrhoea infectious diarrhoea difficult diarrhoea pouchitis |
| 7 | World gasterology organization | Acute diarrhoea anti biotic associated diarrhoea nosocomial diarrhoea adjuvant therapy for H. pylori eradiation irritate bowel syndrome |
| 8 | Malaysian paediatric association | Acute gastroenteritis |
Table 4: Global recommendations.
L. rhamnosus GG: Safety approval
• Lactobacillus rhamnosus GG (ATCC 53103) has a safe history of use in since 1990.
• Qualified Presumption of Safety (QPS) status from European Food Safety Authority (EFSA) scientific committee.
• U.S. Food and Drug Administration (FDA)-GRAS certificate (Figure 5).
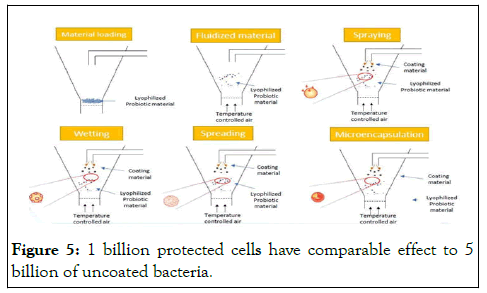
Figure 5: 1 billion protected cells have comparable effect to 5 billion of uncoated bacteria.
Comparative clinical study
Comparison of the kinetics of intestinal colonization by associating 5 probiotic bacteria assumed either in a microencapsulated or in a traditional uncoated form [16].
Study design: Randomized, double blind cross over study.
Treatment
Group A: Mix. of 5 probiotic strains (L. acidophilus LA02, L. rhamnosus LR04, L. rhamnosus LR06, L. rhamnosus GG (ATCC 53103) and B. lactis BS01 each strain 5 billion CFU/day (total 25 billion/day) in uncoated form (n=27).
Group B: Same strains each 1 billion CFU/day (total 5 billion/ day) in microencapsulated form (n=26).
Duration: 21 days treatment followed by 21 days washout period and again 21 days.
Comparable colonization of Lactobacillus rhamnosus
Comparable colonization of L. rhamnosus GG in microencapsulated Lactobacillus rhanmnosus GG group (1 billion per day) and uncoated Lactobacillus rhanmnosus GG group (5 billion per day) (Figure 6 and Table 5).
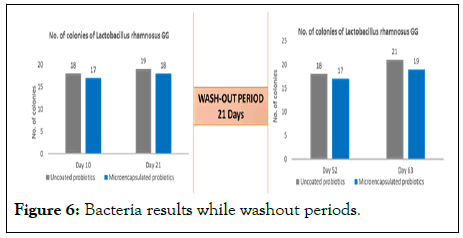
Figure 6: Bacteria results while washout periods.
Challenges in probiotics
• Side effects
• Antibiotic resistance transfer
• Safety
• Heterogeneity and product quality.
| Parameter | Product 1 | Product 2 | Product 3 |
|---|---|---|---|
| Species on label | Bacillus clausii | Bacillus clausii | Bacillus clausii |
| Isolated species | Bacillus clausii | Bacillus subtilis | Bacillus subtilis |
| Label count | 2 × 109 | 2 × 109 | 2 × 109 |
| Isolated species count | |||
| Batch 1 | 1 × 109 | 4 × 106 | 5 × 109 |
| Batch 2 | 2.5 × 108 | 2.5 × 106 | 2 × 108 |
| Batch 3 | 1 × 109 | 1.6 × 106 | 7 × 108 |
| Batch 4 | 2 × 109 | 3.4 × 106 | 1.3 × 108 |
Table 5: Product parameters.
Product 2 (Tufpro-expiry range: 01/18 -08/18) and product 3 (Darolac aqua-expiry range: 12/17-09/18). The expiry date of products as mentioned by manufacturer was two years for all three products. MALDI-TOF-MS identification method (Matrix Assisted Laser Desorption Ionization Method Time of Flight- Mass Spectrometry) was used to identify bacterial species. Plate count method was used and colonies were counted as Colony Forming Units (CFU)/sample using Miles and Mishra method. Only product 1 was found to contain a homogenous population of Bacillus clausii, whereas product 2 and product 3 showed growth of Bacillus subtilis species in the samples. None of the samples had uniform viable bacterial counts across all samples as mentioned on the labels.
Content analysis of commercially available probiotics
We carried out content analysis of four batches each of 3 commercially available probiotic formulations of Bacillus clausii. Species identification was done using MALDI-TOF-MS technique. While bacterial count was done using plate colony count. Only one of the three probiotic formulation analyzed was found to have homogeneous population of B. clausii while none was found to have the exact viable bacterial count as suggested on the label.
World Health Organization (WHO) defines probiotics as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. The pharmaceutical market is flooded with numerous probiotic products with very few effective checks-and-balances to regulate their study were carried out between September 2016 to January 2017. After hospital's ethical committee approval. We tested a total of 12 samples (4 each-4 different batches) of 3 popular probiotics containing B. clausii namely-CRE cy rove: 05/12. 03/19).
L. rhamnosus GG significantly reduced duration of diarrhoea, daily stool outputs, improved stool consistency and reduced no. of hospitalization and fever in children compared to other probiotics (B. clausii and S. boulardii) and ORS group.
None.
None.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hawal AIM, Hussin FET, Gad SMH, Mohamed GIM (2023) Recent Emerging Gut Microbiota Management Modalities in Acute Cases of Diarrhea in Children: A Comparative Study Review about Use of Probiotics. J Prob Health. 11:330.
Received: 09-May-2023, Manuscript No. JPH-23-25131; Editor assigned: 12-May-2023, Pre QC No. JPH-23-25131(PQ); Reviewed: 26-May-2023, QC No. JPH-23-25131; Revised: 14-Jun-2023, Manuscript No. JPH-23-25131(R); Published: 29-Sep-2023 , DOI: 10.35248/2329-8901.23.11.330
Copyright: © 2023 Hawal AIM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : None