Autism-Open Access
Open Access
ISSN: 2165-7890
ISSN: 2165-7890
Research - (2020)Volume 10, Issue 2
Autism Spectrum Disorder is a developmental disorder that affects communication and behaviour. Children with ASD are 4 times more likely to experience gastrointestinal symptoms as compared to children without ASD due to less diversity of bacteria in their gut. The gastrointestinal dysbiosis has been considered as the most important contributing factor of increasing GIT symptoms among children with ASD. Evidence suggested that alteration in the intestinal micro flora is connected with the severity of ASD symptoms. Probiotic therapy has been proposed as a therapeutic tool for augmented gastrointestinal symptom severity among children with ASD. This narrative review systematically searched the literature to provide an update to Teachers and Nutritionist about the therapeutic role of probiotics to manage the children with ASD by reducing gastrointestinal symptoms. A total of 200 articles were screened and 5 articles met the inclusion criteria. There is promising evidence to suggest that probiotic therapy may improve gastrointestinal dysbiosis and reduce the severity of the ASD symptoms among children with ASD. Further research is needed in this context, to recommend about dosage and duration of treatment.
Probiotics; Autism; Gastrointestinal symptoms; Dysbiosis
Autism spectrum disorder (ASD) is a complex developmental disorder that is characterized by deficits in social and communicative behaviour as well as repetitive patterns of behaviour [1]. In May 2013, the fifth edition of the American Psychiatric Associations Diagnostic and Statistical Manual (DSM-V), merged five disorders as pervasive development disorders or autism spectrum disorders into one umbrella diagnosis of ASD included autism disorder (classic autism), Asperger’s disorder (Asperger’s syndrome), Pervasive development disorder not otherwise specifies (PDD-NOS), Rett’s disorder (Rett’s syndrome) and Childhood disintegrative disorder (CDD). Previously they were recognized as distinct subtypes [1]. The prevalence of ASD has more than doubled from 1 in 150 as of 2000 to 1 in 59 as of 2018 in the United States of America, with a similar trend worldwide [2]. Although India is the second most populous country of the world, a large portion of the population of this country is below 20 years of age but still there is a paucity of information about the prevalence and incidence of Autism and other developmental disorders [3]. It is also estimated that ASD is 4.5 times greater among boys than girls [2]. Its etiology is difficult to find and it’s thought to involve a combination of genetic changes and environmental factors [4,5].
According to American Psychiatric Association, 2000 symptoms are evident in children with autism prior to age of 3. Symptoms that are associated with ASD include impairments in social– emotional reciprocity, non- verbal communication behaviours and the development and maintenance of relationships as well as stereotypical repetitive behaviours [1]. Along with these, neurodevelopment concerns gastrointestinal symptoms are also commonly reported among children with ASD, including diarrhoea, constipation or in combination [6-8]. In one of the study, it was pointed out that, children with ASD were 4 times more susceptible to experience gastrointestinal symptoms as compared to children without ASD. Diarrhoea is reported to be one of the commonly recognized symptoms and be easily affecting well-being of children with ASD [6-11].
Some of the commonly reported maladaptive behaviour among children with ASD due to gastrointestinal dysfunction such as sleeps disturbances, aggressive behaviour, irritability or selfinjury that ultimately affects the quality of life of the child and simultaneously affects parental and family stress levels [12]. The cause of the gastrointestinal dysfunction in children with ASD has not been definitely determined but there is some evidence that gut micro biota may play a role [13,14]. Along with this, Leaky Gut Syndrome i.e., inflammation of intestinal lining or increased permeability, it clearly defines how the dysfunctioning of gut affects the brain functioning. The healthy gastrointestinal tract absorbs only the small molecules of completely broken down food particles that are the result of fully digested food. Normally, the intestinal wall should have the ability to keep out large and undesirable molecules. But when the ability is reduced, then permeability increases and large spaces develop between the cells of the gut wall allowing for yeast overgrowth, pathogens, toxins, bacteria, viruses and food to leak across the intestinal lining. Now the gut lining is permeable and therefore further inflamed and damaged disrupting the normal functioning of the gastrointestinal tract. The latter may lead to food allergies because immune system treats them as an invader. It can also lead to opioid effect. Proponents believe that partially digested protein molecules from gluten or casein known as peptides can reach the brain by the blood stream. Peptides have a molecular structure similar to that of your brain’s natural opioids (endorphins), so they are attach to the brains opioid receptors. This leads to the problems with behaviour, speech skills [14].
Studies have shown change in the composition of the gut micro biota in children with ASD as compared with neuro-typical controls [12,13,15,16]. These differences consists of a lower relative abundance of several bacteria including the genera bifido-bacterium, prevotella, streptococcus, enterococcus, lactococcus, lactobacillus, ruminococcus, coprococcus and staphylococcus and higher firmicutes to bacterioidetes ratios [12,15,17-20]. The latter of which has preliminary been associated with the severity of ASD [13,20].
Probiotic therapy has been proposed as a treatment for augmented gastrointestinal symptoms severity among children with ASD. Probiotics are living non-pathogenic microorganisms, which beneficially affects the host’s health when given in right amount as supplement. Probiotics products consist mainly of lactic acid-producing bacteria, such as lactobacilli, lactococci and bifidiobacteria or yeasts such as saccharomyces boulardii. The wellestablished effects of probiotics vary with species and particular strains of bacteria chosen [21].
The purpose of this narrative review is to examine the current literature to address if common gastrointestinal symptoms (abdominal pain, gastro-esophageal reflux, diarrhoea and constipation) among children with ASD improve with the provision of probiotics. Noticing the present state of the evidence will provide clear picture to the teachers and nutritionists whether or not probiotics may benefit their clients with ASD who are experiencing gastrointestinal symptoms and can guide them in addressing about the therapeutic role of probiotics to the parents and professionals.
Autism is noticed firstly within the first few years of life, it is considered a complex developmental and functional disorder. Diagnosis of autism has increased tenfold in the last 40 years. Through assessment the researcher noted that children with Autism are more likely to experience gastrointestinal dysfunction as compared to children without autism due to dysbiosis of gut micro biota or leaky gut syndrome. Hence, it was found essential for reviewing the therapeutic role of probiotics to manage the gastrointestinal dysfunction among children with ASD and its connection with core ASD symptoms.
The aim of this narrative review is to examine and analyse the therapeutic role of probiotics to manage the gastrointestinal dysfunction among children with ASD.
This narrative review was conducted with the (PRISMA) Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. A comprehensive strategy was performed using the following databases PubMed, CINAHL and SCOPUS with the use of keywords and Mesh terms “Probiotics”; “Prebiotics”; “Symbiotic”; “Lactobacillus”; “Autism”; “Pervasive Developmental Disorder” and “Aspergers” to locate pertinent information published in English over the last 10 years. Figure 1 provides details on the steps of the search strategy. The search was completed till 21 November 2019 and studies that included children with ASD less than or equal to 18 years. All relevant titles and abstracts were read to assess the eligibility based on the inclusion criteria. After reading the full texts, the researcher removed non-relevant articles where potentially relevant articles were evaluated by the other author to confirm eligibility. Initial search retrieved 200 articles, over which 67 articles were excluded because of duplication in the databases, remaining titles and abstracts were screened and 93 articles again excluded as they were related to other non–clinical papers and some are based on animal studies. Remaining 40 articles were met the inclusion criteria but only 4 studies met the objective of the review and additional 1 article identified by the hand search or other methods. So, total 5 articles were included in this narrative review. Reference lists of the relevant articles as well as pertinent reviews were screened to identify additional articles for inclusion. Although, the available literature was limited to explain much about the connection between the dysfunction of gastrointestinal tract and severity of ASD among children with ASD. There have only been 5 published research papers to our knowledge that describe the effect of probiotic therapy on gastrointestinal dysfunction among children with ASD.
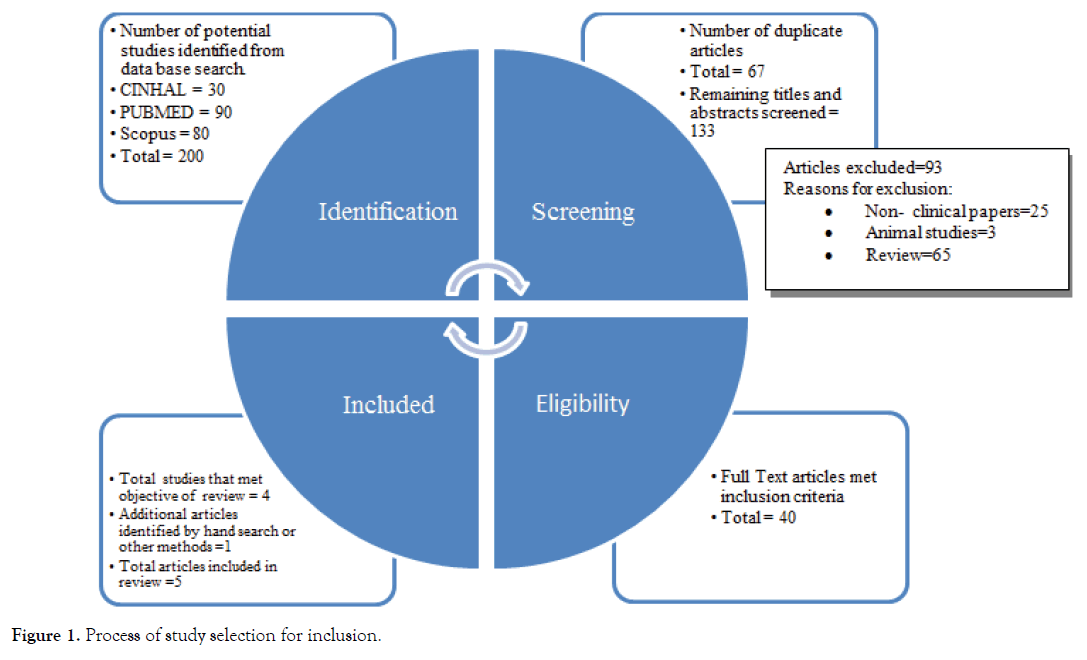
Figure 1. Process of study selection for inclusion.
Criteria used for ASD Diagnosis:
• ASD (ICD-10 criteria)
• ASD (DSM-V)
• ASD (DSM-IV criteria)
Study Design:
• Prospective, open–label non controlled trial
• Non controlled trial
• Double-blind, Placebo-controlled, Crossover trial.
Types of Participants:
• Non- ASD siblings
• Children with ASD
• Non-ASD controls
Articles included in this narrative review from 2010 onwards.
A detailed explanation of the five studies is given in Table 1. The sample size ranged from 22–60 participants.
| Shaaban (2017) | Tomova (2015) | West (2013) | Kaluzna-Czaplinska & Blaszczyk (2012) | Parracho (2010) | |
|---|---|---|---|---|---|
| Location | Egypt | Slovakia | USA | Poland | United Kingdom |
| Research Design | Prospective, open label Non-controlled trial | Non-controlled trial | Non-controlled trial | Non-controlled trial | Double- blind, placebo-controlled, crossover trial |
| Type of participants & Age | 60 children (5-9 years) (30 ASD & 30 controls) | 29 children: 10 ASD (2-9 years), 9 non ASD siblings (5-17 years), 10 non-ASD controls (2-11 years) | 33 children (3-16 years) with ASD | 22 children (4-10 years) with ASD | 22 children (8-9 years) with ASD |
| Sampling technique | Convenience sampling | Not reported | Convenience sample | Not reported | Not reported |
| Sex | 19 boys & 11 girls | ASD (90%Males), Non-ASD siblings (77.7%) males, Non-ASD controls (100%) males | Not reported | 91% Males | 91% Males |
| Strain of Probiotic | 3 strains: Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacteria longum |
“Children Dophilus”: Blend of: 3 strains of Lactobacillus (60%), 2 strains of Bifidumbacteria (25%), 1 Strain of Streptococcus (15%) (exact strain information not provided) |
Delpro capsule: 2 billion CFUs of each of the following: Lactobacillus delbrueckii, L. acidophilus, Lactobacillus casei, B. longum, Bifidobacteria bifidum; with an additional 8 mg Del-immune V powder |
L. acidophilus | Lactobacillus plantarum |
| Dosage and duration of Probiotics therapy | 5 gm/day & 1 time/day for 3 months | Dosage information not provided & 3 times/day for 4 months | 1 capsule, 3 times/day for 21 days |
1 capsule, 2 times/day for 2 months | 1 capsule, daily for 3 weeks |
| Ingestion of food sources of Probiotics & change in dietary habits | Not considered | Not considered | Not considered | Not considered | Not considered |
| Concurrent therapies including (additional Probiotics & dietary supplements) | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated |
| Gastrointestinal symptoms before intervention | GI symptoms assessed including constipation, diarrhoea, stool consistency and smell, flatulence & abdominal pain using 6-GSI | Parents’ questionnaire at baseline only | 84% had moderate or severe constipation& 56% had moderate or severe diarrhoea before intervention (based on ATEC) | No objective reporting. Authors indicated all children had “severe” GI problems (abdominal pain, constipatio, diarrhoea) | Parents recorded GI functions and symptoms in a diary throughout the study |
| Food intake | Not recorded | Not recorded | Not recorded | 45% of participants had a restricted diet and authors noted that all of the children were on a “sugar- free diet” | Not recorded |
| Analysis of behavior | ATEC (Autism Treatment Evaluation Checklist) was administered pre-post intervention | CARS (Childhood Autism Rating Scale) and ADI (Autism Diagnostic Interview) administered only at baseline. | ATEC (Autism Treatment Evaluation Checklist) was administered pre-post intervention | None | Behaviour analysis was done with placebo and Probiotic group using DBC-P tool (Development Behaviour Checklist-Primary carer version) & TBPS (Total Behaviour Problem Score) |
| Results | Children with ASD had lower bifidobacteria levels than controls before intervention and the fecal level of Lactobacillus and Bifidobacteria concentration increased as a result of supplementation(p<0.0001) | Children with ASD and their siblings had more gastrointestinal dysbiosis than control (p<0.05) and gastrointestinal dysfunction was strongly positively correlated with Autism core symptoms | Severe constipation was reported by 52% of the total respondent before intervention and 20% reported after intervention. Severe diarrhoea reported by 20% of the respondents before intervention with a decline to none after treatment | Urine DA to LA metabolites decreased after Probiotic supplementation i.e., from mean of 3.15 ± 0.41 before treatment to a mean of 2.77 ± 0.28 after probiotic treatment. | Probiotic therapy significantly increased the amount of Lcatobacilli/Enterococci and decreased the amount of the Clostridium coccoides found in the stool samples of children with ASD as compared to placebo group |
| Gastrointestinal symptoms improved measured by using 6GSI (including constipation, abdominal pain, flautulence) was strongly correlated to improve autism Severity particularly, speech, language and communication (measured by using ATEC) | After intervention, there was a significant decrease among all ATEC domain including (communication, social, cognitive, physical behaviour) simultaneously found decrease in gastrointestinal symptoms | No difference was found before and after intervention in the gastrointestinal symptoms (noted by diary) | |||
| Limitations | • It was sample size with convenience sampling and non-blinded. • No control over the ingestion of other Probiotic sources, dietary habits and additional therapy. |
• Gastrointestinal symptoms were measured before intervention using parent questionnaire. Possibility of bias here • Behaviour analysis was done only before intervention. So, here we are unable to determine whether or not probiotics influenced ASD symptoms. • No control over diet, medication and additional therapy • No information about sampling technique |
Use of convenience sampling. Without control group | Objective measurement of gastrointestinal symptoms was not done before and after intervention. No information about sampling technique | No information about sampling technique. No control over diet, additional therapy and other probiotic sources |
Table 1. Summary of Research on the Role of Probiotics in Managing Gastrointestinal Dysfunction among Children with Autism.
This narrative review was conducted to find out the evidence regarding the therapeutic role of probiotics in managing gastrointestinal dysfunction among children with ASD and core ASD symptoms. As reported in Table 1, there were five studies, and the study findings were too heterogeneous and allow meta-analysis for accuracy. This is the first narrative review that has been written systematically to our knowledge which searching the evidence for the pertinent information on therapeutic role of probiotics.
Evidence to date summarised that overall there is a paucity of evidence that support the role of probiotics as a supplementation in improving gastrointestinal symptoms and its relation to maladaptive behaviours among children with ASD. The exact mechanism by which probiotics exert potential therapeutic effects is not known but nevertheless it plays an important role in alleviating the gastrointestinal dysfunction by improving gut micro biota and ultimately, improves the core ASD symptoms [13,20,22].
Literature reported that probiotics would help to restore the gut micro biota back to normal levels and also effective to prevent candida colonization in the gut and one of the study found reduced D- arabinitol levels, a metabolite of candida species in the urine of ASD children after probiotic supplementation [23,24]. Another one of the study reviewed reported that a reduction of clostridium species in the stools samples of children with Autism after probiotic supplementation [25]. Children with ASD experience gastrointestinal symptoms and have been shown to have higher levels of gut immune inflammation that is associated with gut dysbiosis [26,27]. Therefore, probiotics could have a role in restoring gut micro biota as well as lowering levels of gut inflammation. From the studies examined, there is some evidence that probiotic supplementation resulted in an improvement of behaviour of the children with ASD [23,25,28]. Summing up, majority of the study reported that probiotic therapy helps in altering the gastrointestinal dysfunction and improving the core ASD symptoms.
The result extended in this review supports that probiotic is helpful in alleviating the gastrointestinal symptoms and the severity of core ASD symptoms. Further, there is a need to aware the parents/ caregivers of children with autism about the benefits of probiotics. Very few published articles evidenced about the therapeutic role of probiotics for children with autism especially, who are experiencing gastrointestinal dysfunction. Therefore, further research is needed to describe the exact mechanism like how the probiotics would be helpful in treating gut dysbiosis for the children with autism using higher methodological qualities to reduce the risk of bias.
• There is no previous narrative review on therapeutic role of probiotics to manage children with autism.
• A systematic search consistent with PRISMA guidelines.
• This narrative review is having a potential limitation that researcher excluded manuscripts of different languages except English and conference studies without full-text.
• Database search was limited.
• Search strategy was refined to address the therapeutic role of probiotics to manage the gastrointestinal dysfunction among children with autism.
• Meta-analysis will give more accuracy to findings.
Based on all 5 studies which included in this narrative review, role of probiotics in managing the gut dysbiosis among children with Autism. After analysing these studies, some valuable points were found:
• Further research is needed in this context, to know about the strains, dosage and duration of probiotic therapy.
• Dietary habits need to be assessed along with probiotic as an intervention.
• Medications, dietary supplements and additional intake of probiotics must be taken into consideration in further probiotics used intervention studies.
• Case study must be done to establish any correlation between gastrointestinal symptoms and behaviour and what effects of probiotics may have on each.
Autism spectrum disorder area diverse group of disorder caused by a complex relationship between genetic and environmental components. The narrative review of available literature provided suggestive evidence regarding the potential role of probiotics in managing children with Autism by improving gut dysbiosis. Further, there is a lack of randomized controlled trials and most studies had a sample size less than (N>50). In addition, there was no standardized probiotics regimen and no information about strains and concentration of probiotics and duration of probiotics treatment. Looking this, there is a further need of trials based studies along with proper sample size and control group to know about the bacterial strains as effects of probiotic bacteria can be highly strain specific.
Citation: Agarwal T, Asthana N (2020) Probiotics: A Therapeutic Tool to Manage the Children with Autism Spectrum Disorder. Autism Open Access 10: 248. doi: 10.35248/2165-7890.20.10.248.
Received: 30-Jan-2020 Accepted: 28-Feb-2020 Published: 06-Mar-2020 , DOI: 10.35248/2165-7890.20.10.248
Copyright: Copyright: © 2020 Agarwal T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.