
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 3
Increasing evidence suggests that platelets play a critical role in tumor development but the mechanism of platelet tumor interactions is not clear.
This study aims to investigate the effect of platelet tumor cell interactions on Hepatocellular Carcinoma ( HCC) metastasis. The results showed that the invasive migration ability of Huh7 was significantly enhanced after coincubation of cells with platelets derived from HCC patients and healthy controls. Moreover, the apoptosis of Huh 7 was reduced after co-incubation. In addition, the expression of Wnt-β-catenin related proteins was elevated after coincubation. Therefore, we suggest that platelets promote the growth and metastasis of HCC cells through βW- ntcatenin signaling pathway.
Platelet; Interaction; Huh7; Wnt-β-catenin; Clinical laboratory
Hepatocellular Carcinoma (HCC) accounts for 90% of primary liver cancers. HCC is the sixth most common cancer worldwide and the third leading cause of cancer related deaths. Despite many advances in the treatment of HCC, the difficulty of early diagnosis and the high rate of recurrence and metastasis are the primary reasons for the high mortality rate of HCC patients. Therefore, a comprehensive elucidation of the molecular mechanisms of hepatocarcinogenesis and development is important to improve the outcome and prognosis of HCC [1].
Platelets are the smallest blood cells in the circulatory system and their primary role is to maintain hemostasis. They do not have a nucleus, but have a large number of granules containing molecules that are involved in many important physiological processes. These granules can be released upon platelet activation and therefore platelets are involved in hemostasis, wound repair and immunological processes. In addition, platelets are related to the pathophysiogenesis of a number of diseases, including cancer. Platelets can play a part in promoting multiple steps in the progression of tumorigenesis, such as promoting tumor growth, angiogenesis and metastasis [2]. Uncontrollable cell proliferation, invasion and metastasis are essential features of malignancy, and metastasis leads to many cancer related deaths. Cho MS, et al., observed that platelets increased the proliferation of ovarian cancer cells in vitro and in vivo. Similarly, Carr BI, et al., demonstrated that human platelets enhance the growth and invasion of HCC cell lines. Platelets promote adhesion or prevent circulating tumor cell death by forming a physical barrier around tumor cells thereby promoting tumor metastasis. Protects tumor cells from natural killer cellmediated lysis. However, the molecular mechanisms through which platelets promote tumor metastasis have not been fully explored [3].
The evolutionarily conserved developmental process known as Epithelial Mesenchymal Transition (EMT) is linked to the onset of cancer and gives cancer cells the ability to spread to other organs by increasing their aggressiveness and motility [4]. In the realm of cancer treatment, there has been a lot of interest in the direct contact between platelets and tumor cells to encourage EMT. Labelle, et al., demonstrated that direct interaction between platelets and tumor cells results in EMT transition and accelerated metastasis. Direct contact between platelets and breast cancer cells facilitate breast cancer metastasis through integrin α2β1 contact and Wnt-β-linked protein activation.
Therefore, we investigated how platelets affected human HCC cell lines in vitro invasion, migration, and cell proliferation. It was further investigated whether platelets affect the changes of the Wnt-β-catenin pathway in hepatocellular carcinoma cells. Elucidating the role of platelets in liver cancer progression and providing new therapeutic strategies for liver cancer treatment [4].
Cell lines
The human HCC cell line Huh7 was purchased from the liver cancer institute of Shanghai Fu-dan University. High glucose Dulbecco's Modified Eagle's Medium (DMEM) with 10% Fetal Bovine Serum (FBS) was used for Huh7 cell culture and routinely cultured in a 37℃, 5% CO2 incubator [5].
Isolation of human platelet
Blood specimens were collected from healthy volunteers and HCC patients at first affiliated hospital of Guangxi medical university. Collect 10 ml of whole blood from normal healthy individuals and patients with HCC and centrifuge at room temperature, 120 g for 20 minutes. Aspirate the supernatant into another centrifuge tube and centrifuge at room temperature at 360 g for 20 minutes [6]. Remove the supernatant and add hepe-tyrode buffer to suspend the precipitate. Centrifuge at 1000 rpm for 10 min at room temperature, remove the supernatant. Suspend the precipitate with 2 ml of serum free medium, and set aside at room temperature for 30 min. Ethical approval for the experiments in this study was obtained from the ethics committee of the first affiliated hospital of Guangxi medical university [7].
Transwell assay
Cell invasion and migration was assessed with a transwell insert chambers (pore size, 8 μm; Corning, Inc.). For the invasion assay, matrigel was mixed with DMEM serum-free medium at a 1:8 dilution and spread in the upper chamber of the transwell and placed in a 37°C incubator for 3 hours to allow the matrigel to solidify. Briefly, the upper chamber was inoculated with 200 μl of serum-free DMEM containing cells and platelets, and the lower chamber was filled with 600 μl of DMEM supplemented with 10% fetal bovine serum. After incubation at 37°C for 24 h, cells that were migrating and invading were stained for 20 minutes at room temperature after being fixed with 4% paraformaldehyde for 30 minutes. Cells were observed under a 100 x microscope from three fields of view, cell invasion rate was analyzed by ImageJ [8].
Apoptosis assays
Huh7 cells seeded in a 6-well plate at 5 × 105 cells/well for 12 h of routine culture, add platelets and process for 24 hours, the cells were digested with trypsin and collected by centrifugation. The cells were stained according to the instructions of Annex in V-FITC/PI apoptosis kit, and apoptosis was detected by flow cytometer. The experiment was replicated three times [9].
Micro scratch tests
Huh7 cells inoculated in 6-well plates and routinely cultured for 24 h. When cell fusion reached about 90%, the monolayer of cells is scored vertically with a 10 μl pipette tip, remove the residual cells and wash the cells twice with PBS. Divided into solvent control group (serum free DMEM) and experimental group containing healthy control platelets and HCC platelets. The scratch time point was recorded as 0 h, and the cell migration was recorded by taking pictures at 0 h and 24 h. The scratching experiment was repeated three times. The closure rate of scratches was analyzed using Image J.
Western blot
Cells were washed 2 times-3 times with pre-cooled PBS and lysed with RIPA lysing buffer containing protease inhibitors (Solar bio). The supernatant protein concentration of the protein lysate was detected with BCA protein assay kit (Applied Technologies Inc.). The proteins were denatured by adding 5 X Loading Buffer and boiling for 10 minutes. Transfer the proteins after SDS-PAGE electrophoresis onto Poly Vinylidene Difluoride (PVDF) membranes. Incubate overnight with 1:1000 primary antibody and 2 hours at room temperature with 1:5000 secondary antibodies. Finally, the membranes were developed with a Chemiluminescence kit [10].
Statistical analysis
Statistical analyses were performed using SPSS 20.0 and student's t-test or one-way ANOVA. Graph pad prism 8 was used for picture plotting. Measured data are expressed as mean ± Standard Deviation (SD). P-value<0.05 was considered statistically significant [11].
Platelet promoted Huh7 migration and invasion
To evaluate platelet impact on Huh7 cells' capacity for invasion and migration, we use the trans well invasion assay. Results of the scratch assay showed that when Huh7 cells were co-incubation with platelets, there were a significant increase in cell migration and a significant increase in wound healing ability (Figure 1). The effect of platelets on invasive cell migration was further confirmed by trans well migration assay. Similar to the results of the scratch test, platelets coincubation with Huh7 cells increased the number of cells migrating to the lower side of the trans well. Similarly, the invasion assay also showed a significant increase in invasion after co-culture of Huh7 cells with platelets (Figure 2) [12].
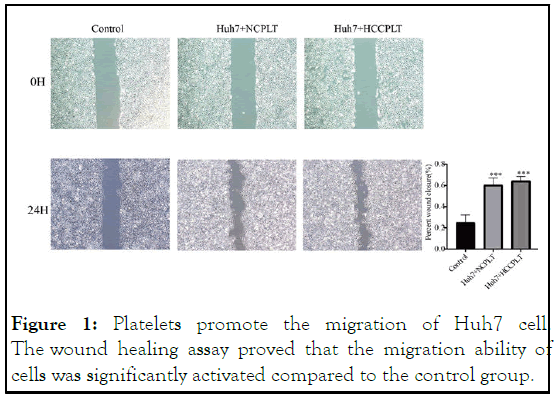
Figure 1: Platelets promote the migration of Huh7 cell. The wound healing assay proved that the migration ability of cells was significantly activated compared to the control group.
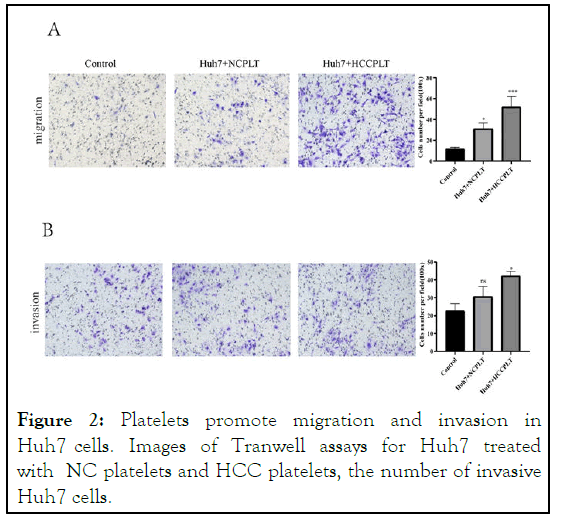
Figure 2: Platelets promote migration and invasion in Huh7 cells. Images of Tranwell assays for Huh7 treated with NC platelets and HCC platelets, the number of invasive Huh7 cells.
Effect of platelets on Huh7 apoptosis
The effect of platelets on Huh7 apoptosis was detected by Annex in flow cytometer, V-FITC/PI Apoptosis detection kit China according to the manufacturer's instructions. These results indicated that the apoptosis rate of Huh7 cells was significantly reduced after 24 h of co-incubation with platelets from healthy controls (Figure 3). However, co-incubation with platelets from HCC patients for 24 hours did not affect the apoptosis of Huh7 cells [13].
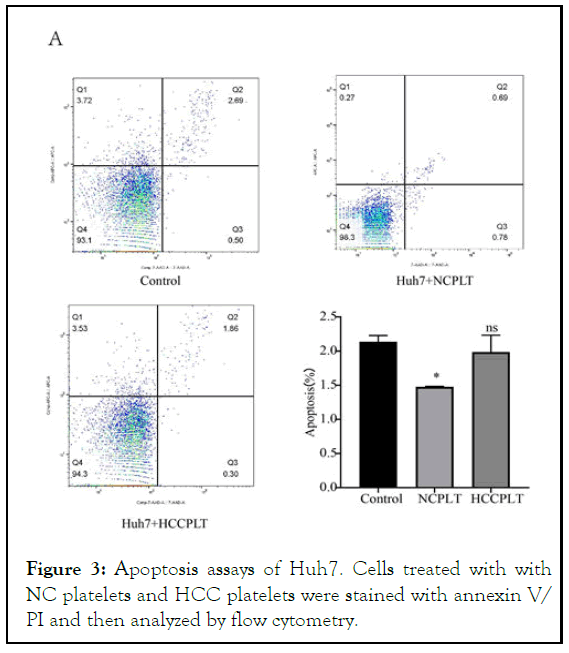
Figure 3: Apoptosis assays of Huh7. Cells treated with with NC platelets and HCC platelets were stained with annexin V/ PI and then analyzed by flow cytometry.
Platelets activate Wnt-β-catenin pathway in Huh7
To further investigate the role of co-incubation with platelets in the Huh7 cell pathway, the expression levels of β-catenin, GSK, P-GSK, CMYC were detected by western blot. Compared with the blank group, β-catenin and phosphorylated GSK, important molecules on the Wnt-β-catenin pathway, were significantly increased at the protein level, while c-Myc, a downstream effector molecule of the Wnt-β-catenin pathway, also showed an increasing trend (Figure 4). Alterations in Wnt-β-catenin pathway associated proteins were observed after 24 hours of co-incubation [14].
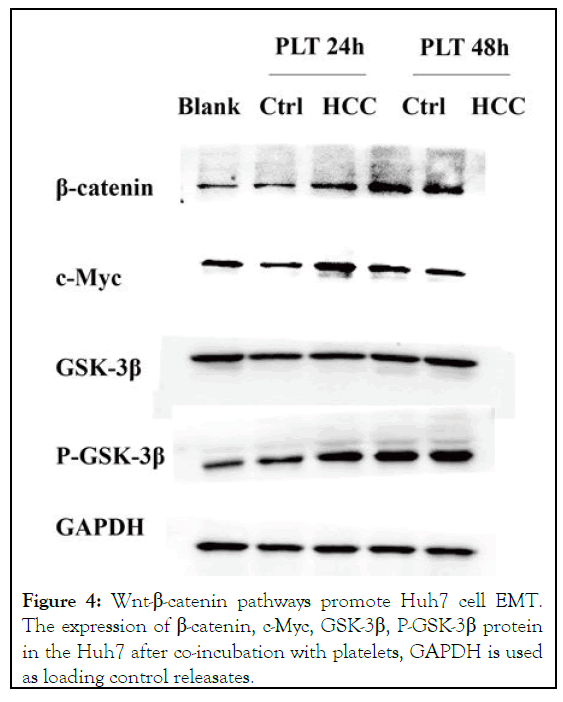
Figure 4: Wnt-β-catenin pathways promote Huh7 cell EMT. The expression of β-catenin, c-Myc, GSK-3β, P-GSK-3β protein in the Huh7 after co-incubation with platelets, GAPDH is used as loading control releasates.
Platelets are essential players in the regulation of tumor metastasis and angiogenesis. Many researchers believe that platelets have a role in promoting metastasis in various types of tumors, such as cholangiocarcinoma breast cancer colon cancer and ovarian cancer. Reported mechanisms of platelet-tumor action include enhances coagulation and alters cell adhesion, produces platelet derived inflammatory cytokines and antigenic factors, etc. In HCC, Brian I Carr, et al., reported that normal human platelet extracts promoted the growth, migration and invasion of human HCC cell lines in vitro. Consistent with the results of previous studies. Our results demonstrated that both platelets from healthy controls and tumor patients not only enhances cell migration and invasion but also the in vitro development of human Huh7 cell lines. Direct contact between platelets and tumor cells induces EMT like transformation and invasive behavior in vitro, and promotes tumor extravasation and metastasis in vivo. In addition, the apoptosis rate of hepatocellular carcinoma cells was markedly decreased after healthy control platelet co-incubation. Analogous to previous studies, co-incubation with platelets resulted in a majority of HCC cells in S-phase and reduced apoptosis. Western blot analysis shows that co-incubation with platelets increase β- catenin, c-Myc, P-GSK-3β levels. Most importantly, our study suggests that co-incubation with platelets for 24 hours caused alterations in the Wnt-β-catenin related proteins and these effects are time-dependent. Wnt-β-catenin signaling pathway is closely associated with the promotion of EMT in HCC cells. We speculate that platelet-HCC cell interactions may exert their pro-metastatic effects through Wnt-β-catenin signaling pathwaymediated EMT [15].
Cancer cells are protected by platelets during the tumor growth and metastasis. Circulating Tumor Cells (CTCs) evade surveillance by the immune system, while promoting angiogenesis and tumor cell adhesion and invasion. Thrombocytosis is significantly related to cancer metastasis. Clinically, high platelet counts are common in cancer patients, and they are typically associated with a poorer prognosis and shorter survival for cancer patients. Therefore, clarifying the molecular mechanism of interaction between platelets and cancer cells can provide novel targets for anti-cancer drug development.
According to our research, platelet-HCC cell interactions are crucial for tumor metastasis. Meanwhile, platelets can promote EMT in HCC through the Wnt-β-catenin signaling pathway. Our study provides a novel target for the treatment of HCC.
This research was supported by national natural science foundation of china and key laboratory of early prevention and treatment for regional high incidence tumor, Guangxi medical university, ministry of education.
The authors declare no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Googlescholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Googlescholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Qin X, Huang J, Liang Y, Chen H, Zuojian Hu, Zhang D, et al. (2024) Platelets promote metastasis of hepatocellular carcinoma cells through Wnt-?-catenin signaling pathway. J Clin Lab Med. 7:293.
Received: 10-Nov-2022, Manuscript No. JCCLM-22-20026; Editor assigned: 14-Nov-2022, Pre QC No. JCCLM-22-20026 (PQ); Reviewed: 28-Nov-2022, QC No. JCCLM-22-20026; Revised: 21-Jun-2023, Manuscript No. JCCLM-22-20026 (R); Published: 28-Sep-2024 , DOI: 10.35248/JCCLM.24.7.293
Copyright: © 2024 Qin X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.