Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2018) Volume 9, Issue 5
Keywords: Apple pomace; Polyphenolics; Optimization; Antioxidant activity; Protocatechuic aldehyde
Extraction of bioactive ingredients using critical fluids have attracted considerable attention in recent times because of their environmentally friendliness. Subcritical water extraction technique is capable of replacing methanol, ethanol and acetone, because water is cheaper, non-toxic and readily available [1]. Subcritical water refers to liquid water of temperature between atmospheric boiling point and that of its critical temperature (100-374°C) [2]. The most important property of subcritical water in terms of its extractive efficiency is the variation of its dielectric constant (ε). Water at room temperature and normal pressure is highly polar with extensive hydrogen bonded structure and therefore not suitable for extracting organic or non- polar compounds [3]. Water at room temperature and normal pressure has dielectric constant of approximately 80 and decreases to 27 at 250°C, which is similar to that of ethanol and acetone at 20°C [4,5].
Subcritical water at selected temperatures, has been shown to demonstrate selectivity towards different groups of compounds based on their polarities. Polar compounds are recovered at lower subcritical water conditions, whilst less polar ones are extracted at higher subcritical water temperatures [6]. The higher concentrations of H+ and OH- ions within the subcritical water medium can promote acid- base reactions such as hydrolysis and dehydration at higher temperatures [7,8]. Hemicellulose, proteins and lignin from plant materials at higher temperatures under subcritical water conditions can undergo hydrolysis to produce amino acids and oligomer sugars [8]. The modified viscosity and diffusivity of water under subcritical water conditions enhances disruption of solute- matrix interactions and thereby improving solubility and effective mass transfer and enhancing elution of strongly bound compounds to cell walls [9].
Application of subcritical water to recover bioactives from plant biomass has been investigated [9,10]. Subcritical water with 60% v/v ethanol as co-solvent was employed to recover antioxidant compounds from lyophilized apple pomace sample [11]. Apple pomace a byproduct of apple juice and cider production is a potential source of polyphenolic compounds, carbohydrates, fibre and pectin whose recovery from the biomass creates routes towards value addition to the residue and applications are found in food, animal feed, pharmaceutical, nutraceutical, chemical, biofuels and cosmetic industries [12-14]. Apple pomace is very heterogeneous solid residue consisting of the peels, discarded or pieces of apples, seeds, core, stems and exhausted apple tissue [15]. It is made up of 25-35% of the fresh weight of apples and contains large amount of water, sugars, small amount of proteins with low pH. Approximately 9 × 106 tons of apple pomace are generated worldwide per the number of apples processed annually from about 70 × 106 tons of apples each year [16]. At least 71% of apples cultivated are eaten fresh and approximately 20% is processed into apple juice and cider production. The remaining is used to produce apple purées, spirits, and other apple by-products [17,18]. Recovery of polyphenols, which are bioactive compounds from the apple pomace by fractionating the biomass aligns with biorefining concept at the same time generate valuable feedstock for pharmaceutical and cosmetic applications [12,19,20]. Apples constitute an important source of flavonoids in the diets within Europe and the United States and approximately 22% of polyphenolics consumed are derived from apples [14]. Procyanidins, pholridzin and epicatechins, quercetin conjugates and chlorogenic acids are the major phenolic compounds found in apples [21]. Investigations have revealed that most of the phytochemicals are found in the peels and therefore the polyphenolic content in the apple pomace is expected to be higher than in the juice and varies among different varieties of apples.
The objective of the current study was to evaluate the utility of subcritical water in the extraction of polyphenolic compounds from wet apple pomace without the addition of organic modifiers. Multivariate statistics techniques with application of Design of Experiments (DoE) and Response Surface Methodology (RSM) approach were employed to design extraction procedures to assess efficient solubilization of the apple pomace residue for polyphenolic compounds recovery, and to analyze antioxidant activity of extract as well as composition and structure of the polyphenolic compounds.
Chemicals
All reagents and standards were obtained at highest purity from the suppliers indicated in the methods.
Apple pomace sample
Apple pomace residue comprising of 7 varieties (Brown Snout, Chisel Jersey, Dabinett, Harry Master Jersey, Michelin, Vilberie and Yarlinton Mill) was supplied by Universal Beverages Limited (UBL), a subsidiary company of Bulmers, UK. The pomace sample was very heterogeneous comprising peels seeds, apple flesh and therefore was thoroughly mixed to ensure replicate samples were representative of the population of samples.
Dry weight of all samples were determined using AOCS (American Oil Chemist Society Standard) standard protocol using a laboratory oven (STATUS international, UK) at 103 ± 3°C. The frozen wet apple pomace sample was homogenized for 30 seconds using Moulinex domestic blending machine to minimize variability in batch-to-batch analysis. Portions of the homogenize apple pomace was freeze dried using vacuum freeze dryer (Model number EQ03 by Vacuum and Industrial Products, UK).
Subcritical water extraction of polyphenolics
Polyphenolic compounds from the apple pomace were extracted under subcritical water mediated hydrolysis conditions of 100-200°C using the Parr instrument model (5521) a stainless-steel reactor vessel (300 mL) of 2.5 in diameter with heating jacket and a magnetic stirrer at 1240 rpm and integrated cooling system. A back pressure regulatory valve was employed to control internal pressure of vessel and nitrogen gas was used to pressurize the reaction vessel to 50 bar. Crude extracts after subcritical water extraction were collected from the reaction vessel and centrifuged using Beckman J2-20 centrifuge. Supernatant were collected and analyzed for total phenolic content and antioxidant activity by Oxygen Radical Antioxidant Activity (ORAC) assay and in High Performance Liquid Chromatography (HPLC).
Total phenolic content
Total phenolic content of all extracts were determined by the Folin Ciocalteus’s micro scale method previously described by Waterhouse [22] using Gallic acid as standard. An aliquot of sample (20 μL) was pipetted into a test tube followed by addition of 1.58 mL of distilled water. Folin- Ciocalteu reagent (100 μL) was then added and shaken to mix. Sodium Carbonate solution (300 μL) added to the mixture and thoroughly shaken by employing Miximatic Vortex. Samples were left for 30 minutes at 40°C in the Clifton Unstirred water bath. After the incubation period, 300 μL of the resulting solutions were pipetted into 96 Well F/B microplate and absorbance read using Promega Glomax Microplate spectrophotometer at 750 nm.
Results were reported in mg Gallic acid equivalents per gram dry weight of apple pomace.
Antioxidant activity by ORAC assay
Oxygen Radical Antioxidant Activity (ORAC) of all extracts were determined using a protocol already published in literature with slight modifications [23]. The fundamental principle of the assay was the inhibition of the oxidation of Sodium Fluorescein (protein target) by a reactive oxygen species relative to Trolox. Fluorescein working solution (150 μL) was added into a 96 well microplate in quadruplicate using a multichannel pipette. Aliquot (25 μL) of control, blank, sample and TROLOX were added into the respective wells. 50 μL of Phosphate buffer (pH 7.4) was added only into the control wells. The solutions in the wells of the microplates where thoroughly mixed using the thermoshaker at 1000 rpm for 3 minutes and allowed to incubate at 37°C for 30 minutes. Then 25 μL of previously incubated 2,2l -azinobis (2-amidinopropane) dihydrochloride (AAPH) at 37°C solution was then added to the blank/sample/TROLOX. wells using a multichannel pipette and mixed thoroughly using the thermoskaker at 1000 rpm for 20 seconds to start the reaction. The microplate was placed into the Promega Microplate reader to record the fluorescence decay per minute for 45 minutes. ORAC data analysis was done using area under the curve of blank/sample/TROLOX in Microsoft Excel 2010 and were obtained by comparing the area under the curve of the intensity of fluorescence overtime of samples and Trolox relative to blank. The results were reported in μmol of Trolox equivalents per gram of dry weight apple pomace.
Experimental design
The experimental design composed of One-Factor at a Time (OFAT) and the actual design by Response Surface Methodology (RSM) using the Central Composite Rotatable Design (CCRD). OFAT experiments investigated the nature of the apple pomace (dried and wet) and the impact of solid-to-solvent ratio, temperature and residence time on overall recovery of polyphenolic compounds as well as antioxidant activity under the subcritical water mediated extraction.
One-factor at a time (OFAT) experiments: The effect of the nature of apple pomace on subcritical water extraction of phenolic compounds was investigated. Both wet and freeze-dried homogenized apple pomace sample were used for solid-to-solvent ratio (1-8% w/v) loading under the subcritical water extraction at a fixed temperature of 150°C for 20 minutes’ residence time. The sample (either dried or wet) that resulted in higher total phenolic content in mg GAE /g dw of apple pomace was selected.
Temperature effects on subcritical water extraction of apple pomace for phenolic compounds was tested using the 1% solid-tosolvent loading for the best sample form at 20 minutes’ residence time. Extractions were carried at 100°C, 160°C and 200°C. Temperature condition that yielded highest total phenolic content in mg GAE /g dw of apple pomace was selected.
Effects of Residence Time on subcritical water extraction of apple pomace phenolic compounds was investigated with 1% solid-to solvent loading at 200°C for 0, 10,20,30 and 60 minutes. The residence time with highest total phenolic content in mg GAE /g dw of apple pomace was selected.
Central Composite Rotatable Design (CCRD) by response surface methodology: The operating limits of the independent variables from the OFAT experiments were used with the aid of the Stat-Ease design software 7.0 to design the experiment for optimization using the CCRD model. Three independent variables (solid-to-solvent ratio, temperature and residence time) were employed in the design to generate 20 experimental runs consisting of 14 trials and 6 replicates around the centre point. The response variables were, total Phenolic content in mg GAE /g dw and ORAC values in μmol of Trolox equivalents per g of dry weight apple pomace.
All experiments were carried out in triplicates and results reported as mean ± standard error. One-way analysis of variance (ANOVA) testing was conducted at 95% confidence level.
Separation and identification of polyphenolic compounds
Phenolic compounds in the subcritical water extracts were separated using Agilent 1100 series HPLC equipment in a reverse mode using a protocol previously described by Schieber [24]. The stationary phase was Prodigy 5 μm ODS3 100A, C18 (250 × 4.6 mm I.D) column from Phenomenex (Torrance, CA, USA) operated at 40°C with a guard column. The mobile phase consisted of 2% (v/v) of the glacial Acetic acid in water as eluent A and 0.5% of Acetic acid in 50:50 (v/v) of Acetonitrile and Water as eluent B. Eluent C was (100%) Acetonitrile. Gradient solvent system was employed with flow rate of 1 mL / min and 10 μL injection volume of all samples. Phenolic compounds were monitored at 280 nm (flavanols), 320 nm (Hydrocinnamic acid) and 370 nm (Flavonols). The compounds were separated depending on their respective affinities between the mobile and stationary phases and were identified by comparing retention times and spectral data using external standards at their maximum absorbance. The external standards comprising, Chlorogenic acid (≥ 95%), (-) Epicatechin (≥ 90%), ± Catechin hydrate, Phloridzin dihydrate (≥ 99%), Procyanidin B2 (≥ 90%), Quercetin -3-β-D-glucoside (≥ 90%), Quercetin-3-Dgalactoside (≥ 97%), and 3,4-Dihydroxybenzaldehyde (≥ 97%) were purchased from Sigma-Aldrich (UK).
The dry matter content of the homogenized apple pomace was 26.2 ± 0.1 g/100 g fresh weight. Dry matter content of apple pomace reported in the literature was 26.4 g/100 g fresh weight [25].
OFAT experiments
The four (4) factors including nature of apple pomace (wet or dried), solid-to-solvent ratio, temperature and residence time were evaluated in the one-factor at time (OFAT) experiment to identify levels of influence of these parameters in terms of recovery of polyphenolic compounds, for selection in an overall experimental design. Selection was based on the factors which resulted in highest total phenolic content expressed in mg GAE/g dw.
The variation of Total phenolic content for dried and wet homogenized apple pomace samples at 150°C for 20 minutes’ residence time are shown in Figure 1.
Analysis of variance (ANOVA) was conducted for total phenolic content (TPC) of the wet and dried apples pomace in mg GAE /g dw using XLSTAT-2014.02 statistical package with post hoc Tukey test. There was no significant difference in total phenolic content between corresponding 1%,2% and 4% of wet and dried samples (p>0.05). Therefore, the number of phenolic compounds that can be extracted using 1% solid loading of wet and dried apple pomace would be similar and so for 2% and 4% loadings.
Total phenolic content (mg GAE /g dw) however differed for 6% and 8% loadings between wet and dried apple pomace, with slightly higher values recorded for wet samples compared to corresponding dried ones. Drying of the apple pomace promoted changes in physical characteristics such as porosity and shrinking of the size of their particles. Transformation of some of its structural properties may have occurred. These alterations in the properties of the apple pomace due to drying would have affected its uptake of water during the rehydration process, which resulted in lowering the amount of phenolic content in the 6% and 8% of the dried samples. This observation was consistent with similar research findings reported for dried plant materials [26,27]. Rehydration of dried biomass generally involve three processes occurring simultaneously. 1) Absorption of water by the dried biomass; 2) Swelling or expanding of biomass as it takes up water; 3) Elution of the soluble ingredients into the extraction fluid [28,29]. The more solid fractions in the 6% and 8% of dried pomace compared to water, may have delayed the leaching process; hence, lower amounts of phenolic content in extracts was observed.
For higher solid to solvent ratios, the dried apple pomace will always lag behind the wet by a step-in extraction mechanism of the biomass under subcritical water mediated hydrolysis. The mechanism involves solute diffusion from the core material to the surface, which would have to be transferred into the extraction fluid, and finally be removed from the extraction cell [30]. Therefore, dried samples always need rehydration step, before the actives are diffused from the core to the surface depending on the amount of water available. However, if the biomass loading is high without sufficient volume of water as solvent, it may lead to incomplete extraction [3]. Hence, wet homogenized apple pomace was selected and used to lower cost and energy associated with drying.
Effect of temperature: Temperature influence on Total phenolic content from the wet apple pomace was studied under OFAT experiment using 1% solid to solvent ratio for residence time of 20 minutes at 100°C, 160°C and 200°C. Total phenolic content increases from 6.93 mg GAE /g dw to 46.25 mg GAE /g dw as temperature was increased from 100°C to 200°C as shown in Figure 2.
The high temperatures under the subcritical conditions broke the cohesive forces linking the particles within the apple pomace matrix. The related forces include, hydrogen bonding, van der Waals forces, and dipole-dipole interaction. Adhesive and cohesive forces holding the solutes within apple pomace matrix were broken and the solutes were eluted into the subcritical water by simple convective mass transfer [31]. However, increasing temperature far off certain limits would degrade polyphenolic compounds or even generate undesired compounds. Non- enzymatic browning of extract was observed beyond 150°C due to caramelisation of sugars in the extract [32]. Although antioxidant compounds could possibly be extracted at higher temperatures, however this condition was not suitable. Higher amounts of furfural and 5-HMF were formed and constitutes possible agents of Maillard reaction which could have toxicological issues in food samples [33]. Similar observations were previously reported [11,34,35]. Hydroxymethylfurfural can metabolise to 5-sulphooxymethylfurfural which to some extent may be harmful. 5-HMF was shown to be initiator and promoter of colon cancer [36], neurotoxicity [37]. Higher amounts of Hydroxymethylfurfural has been reported to be cytotoxic and causes irritation to skin, eyes, upper respiratory tract, and membranes of the mucous [38]. However, data on levels of toxicity of 5-HMF in food samples had not been documented comprehensively [39]. Therefore, estimates for recommended intake are not clear and also no conclusive standards about the dangers to human health due to exposure. Extracts at 200°C were darker and smells different compared to 100°C and 160°C. Therefore, 200°C was selected for the upper limit temperature since it recorded higher total phenolic content of 46.25 mg GAE /g and 100°C was the lower limit.
Effect of residence time: The impact of residence time on total phenolic content was investigated using 1% (solid/solvent) loading at 200°C for residence times 0,20,30 and 60 minutes. Total phenolic content value increased by about 36% for holding time from 0 minute (33.89 mg GAE/g dw) to 20 minutes (46.25 mg GAE/g dw) and remained about the same until 30 minutes as shown in Figure 3.
Results did not show a highly significant difference between total phenolic content at 30 and 60 minutes. Total phenolic content increased by 7.24% moving from 20 to 60 minutes’ residence time. The difference could be attributed to composition of the wet apple pomace used in the extraction. Similar observations using pomegranate seed residue under subcritical water extraction of polyphenolic compounds were made [40]. Their findings revealed there was no difference in total phenolic content for 30 minutes and that of 120 minutes’ extraction times and there was no advantage in recovering polyphenolic compounds beyond 30 minutes. The subcritical water at this temperature would have saturated the apple pomace matrix with sufficient amount of energy thereby eluting the phenolic compounds earlier. Therefore, increasing the residence time beyond 30 minutes at 200°C can lead to degrading of the phenolic compounds that were already eluted earlier [41]. High recovery of antioxidant compounds from grape pomace using pressurized water at 30 minutes’ residence time had been reported. Therefore 10 and 30 minutes residence times were selected and used as the lower and upper limits respectively in the extraction processes.
Experimental design by response surface methodology under subcritical water extraction
Recovery of polyphenolic compounds using subcritical water mediated hydrolysis was influenced by solid/solvent ratio, temperature of extraction and residence time based on the one factor at a time experiment. The lower and upper limits of the independent factors identified in the OFAT exercise were employed to design the experiments using Stat- Ease 7.0.0 design expert (Inc Minneapolis, USA) by response surface methodology using the central composite rotatable design to optimize polyphenolic compounds recovery from the wet apple pomace. In all, 20 experimental points were generated comprising of 14 trials and 6 replicates runs around the centre points. The design summary is shown in Table 1.
| Factor | Name | Units | Low Actual | High Actual | Mean | Std. Dev. |
|---|---|---|---|---|---|---|
| A | Solid/Solvent | % | 1 | 8 | 4.55 | 2.64 |
| B | Temperature | °C | 100 | 200 | 150 | 41.31 |
| C | Residence Time | Min | 10 | 30 | 20 | 8.30 |
Table 1: Design summary under Subcritical Water Extraction (SWE).
Subcritical water extractions were performed for each of the experimental points using the wet homogenized apple pomace with dry matter content measured as 27% and total phenolic content (TPC in mg GAE/g dw) was determined.
Selection of appropriate model for optimization of total phenolic content and antioxidant activity under subcritical water extraction: Many model options including linear, two factor interaction and polynomial models were tested to select the most appropriate one to depict real time response of the surface. Stat-Ease Design Expert statistical tool was used to conduct analysis of variance (ANOVA) on the data. For a model to fit well, it should be significant and have an insignificant lack of fit. Furthermore, the models were contrasted based on adjusted R2 and predicted R2. The success of the regression analysis was not based on the value of coefficient of determination R2 alone, as it is the calculation of the variation explained by the model relative to the overall average of response. As the statement goes “ don’t let R2 value fool you” [42]. The quadratic model was the most appropriate and experimental results were fitted to a quadratic model. In setting up a good model, the simplest one is the most preferred and to achieve this, may require eliminating outliers and conducting transformations of the selected models. Total phenolic content and antioxidant activity values by ORAC followed a generalized transformed second order polynomial as in;
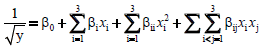
Where, β0 βi βii and βij are the respective coefficient of egression for intercept, linear, quadratic and interaction terms. xi and xj are coded design variables.
The models were highly significant (p<0.0001) and the lack of fit insignificant (p>0.05) with satisfactory level of adequacies (R2>0.9) and predicted R2 largely agreed with adjusted R2. Solid/solvent ratio and extraction temperature had significant effect on Total phenolic content (mg GAE /g dw) and ORAC value (μmol TE/g dw) (p>0.05) for their linear terms, as well as their interaction (p<0.0001). Residence time demonstrated least significance on the responses, consistent with previously reported findings [35]. The results of analysis of variance for Total Phenolic content are shown in Tables 2 and 3 and antioxidant activity by ORAC in Tables 4 and 5.
| Source | Sum of Squares | df | Mean Square | F Value | P Value | |
|---|---|---|---|---|---|---|
| Model | 0.44 | 6 | 0.073 | 285.70 | <0.0001 | significant |
| A Solid/ Solvent | 0.24 | 1 | 0.24 | 932.80 | <0.0001 | |
| B Temp | 0.10 | 1 | 0.10 | 398.90 | <0.0001 | |
| C Res. time | 2.373 × 10-5 | 1 | 2.373 × 10-5 | 0.093 | 0.7671 | |
| AB | 0.049 | 1 | 0.049 | 189.79 | <0.0001 | |
| A2 | 2.449 × 10-3 | 1 | 2.449 × 10-3 | 9.56 | 0.0114 | |
| C2 | 1.413 × 10-3 | 1 | 1.413 × 10-3 | 5.52 | 0.0407 | |
| Residual | 2.561 × 10-3 | 10 | 2.561 × 10-3 | |||
| Lack of Fit | 1.475 × 10-3 | 5 | 2.951 × 10-4 | 1.36 | 0.3724 | Not significant |
| Pure Error | 1.086 × 10-3 | 5 | 2.172 × 10-4 | |||
| Cor Total | 0.44 | 16 |
Table 2: ANOVA for Response Surface Reduced Quadratic Model for Total Phenolic Content under SWE.
| Std. Dev. | 0.013 | R2 | 0.9942 |
| Mean | 0.36 | R2Adj | 0.9907 |
| C.V.% | 4.47 | R2Pri | 0.9742 |
| PRESS | 0.011 | Precisionadeq | 52.475 |
Table 3: Adequacy Level for Response Surface Reduced Quadratic Model for TPC under SWE.
| Source | Sum of Squares | df | Mean Square | F Value | P Value | |
|---|---|---|---|---|---|---|
| Model | 2.95 × 10-3 | 6 | 4.92 × 10-4 | 93.64 | <0.0001 | Significant |
| A Solid/ Solvent | 1.67 × 10-3 | 1 | 1.67 × 10-3 | 318.22 | <0.0001 | |
| B Temp | 6.15 × 10-4 | 1 | 6.15 × 10-4 | 117.07 | <0.0001 | |
| C Res.time | 2.95 × 10-5 | 1 | 2.96 × 10-5 | 5.63 | 0.0451 | |
| AB | 2.43 × 10-4 | 1 | 2.43 × 10-4 | 46.14 | 0.0001 | |
| A2 | 2.004 × 10-5 | 1 | 2.00 × 10-5 | 3.81 | 0.0867 | |
| B2 | 1.26 × 10-4 | 1 | 1.26 × 10-4 | 24.00 | 0.0012 | |
| Residual | 4.21 × 10-5 | 8 | 5.26 × 10-6 | |||
| Lack of Fit | 1.56 × 10-5 | 4 | 3.90 × 10-6 | 0.59 | 0.6899 | Not Significant |
| Pure Error | 2.65 × 10-5 | 4 | 6.62 × 10-6 | |||
| Cor Total | 2.99 × 10-3 | 14 |
Table 4: ANOVA for Response Surface Reduced Quadratic Model for ORAC values under SWE.
| Std Dev | 0.0023 | R2 | 0.9860 |
| Mean | 0.024 | R2Adj | 0.9754 |
| C.V% | 9.65 | R2Pri | 0.9552 |
| PRESS | 0.00013 | Precisionadeq | 30.733 |
Table 5: Adequacy Level for Response Surface Reduced Quadratic Model for ORAC under SWE.
The ratio of signal to noise termed as adequate precision of the analysis was 52.475 for total phenolic content which was good, a value greater than 4 was desirable and therefore the model could be used to navigate the design space.
Coefficient of variation for Total phenolic content determination was 4.07% suggesting a high level of precision and reliability of measured values. Variance Inflation Factors (VIF) describes how much of the variance of the coefficients were inflated as a result of linear relationship with other predictors. VIF has a lower value of 1 with several recommended upper bound values. Maximum value of 10 has been reported [42]. Lower levels of inflation are usually required because higher values of VIF constitute a problem in regression analysis. Variance Inflation Factor (VIF) of solid/solvent ratio of 1.12 meaning the coefficient was larger than by a factor of 1.12 than would otherwise be if no intercorrelations between total phenolic content, temperature and residence time. VIF of all design factors were acceptable as shown in Table 6. Similar results were obtained for antioxidant activity by ORAC.
| Factor | Coefficient Estimate | df | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | 0.35 | 1 | 6.146 × 10-3 | 0.34 | 0.37 | |
| A- Solid/Solvent | 0.16 | 1 | 5.291 × 10-3 | 0.15 | 0.17 | 1.12 |
| B-Temperature | -0.11 | 1 | 5.400 × 10-3 | -0.12 | -0.096 | 1.07 |
| C-Temperature | -1.769 × 10-3 | 1 | 5.812 × 10-3 | -0.015 | 0.011 | 1.29 |
| AB | -0.087 | 1 | 6.703 × 10-3 | -0.01 | -0.073 | 1.08 |
| A2 | -0.021 | 1 | 5.764 × 10-3 | -0.036 | -5.793 × 10-3 | 1.15 |
| C2 | -0.014 | 1 | 6.318 × 10-3 | -0.026 | -6.944 × 10-4 | 1.21 |
Table 6: Coefficient Estimates for factors of Reduced Quadratic Model for TPC under SWE.
The final model equations in terms of actual factors for Total phenolic content (mg GAE /g dw) and ORAC (μmol TE/g dw) are shown below:
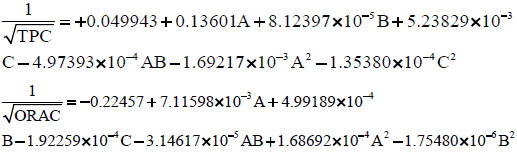
The actual and predicted Total Phenolic content (mg GAE/g dw apple pomace) are shown in the case statistics results in Table 7.
| Solid/solvent (%) | Temperature (°C) | Residence time (min) | Actual TPC Value (mg/g GAE) | Predicted TPC Value (mg/g GAE) |
|---|---|---|---|---|
| 1 | 100 | 10 | 28.2 ± 1.90 | 30.87 |
| 8 | 100 | 10 | 2.2± 0.01 | 2.16 |
| 1 | 200 | 10 | 51.1 ± 1.60 | 51.02 |
| 8 | 200 | 10 | 12.9 ± 0.04 | 11.89 |
| 1 | 100 | 30 | 33.6 ± 0.88 | 30.87 |
| 8 | 100 | 30 | 2.3 ± 0.20 | 2.16 |
| 8 | 200 | 30 | 13.3 ± 0.17 | 11.89 |
| 0.5 | 150 | 20 | 54.3 ± 0.50 | 51.02 |
| 9.5 | 150 | 20 | 3.1 ± 0.08 | 3.43 |
| 4.5 | 150 | 3 | 5.7 ± 0.20 | 3.43 |
| 4.5 | 150 | 37 | 9.3 ± 0.11 | 10.41 |
| 4.5 | 150 | 20 | 7.8 ± 0.06 | 8.16 |
| 4.5 | 150 | 20 | 8.6 ± 0.13 | 8.16 |
| 4.5 | 150 | 20 | 7.0± 0.01 | 8.16 |
| 4.5 | 150 | 20 | 8.7 ± 0.04 | 8.16 |
| 4.5 | 150 | 20 | 7.5 ± 0.17 | 8.16 |
| 4.5 | 150 | 20 | 7.8 ± 0.05 | 8.16 |
Table 7: Case Statistics Report for Actual and Predicted TPC under Subcritical water extraction.
Three dimensional plots (3D) depicting the influence of solid/ solvent ratio and extraction temperature whilst keeping residence time at 20 minutes on the total phenolic content and antioxidant activity by ORAC are represented in Figures 4a and 4b.
Total phenolic content and ORAC values increased with increasing temperature from 100°C to 200°C, however decreasing exponentially as the solid/solvent ratio increases. The curvature of the response plots suggests that, it is unpractical to recover higher amounts of polyphenolic compounds and antioxidant activity by increasing both solid/solvent ratio and temperature of extraction simultaneously. Increase in extraction temperature from 100°C to 200°C at 1% increases total phenolic content from 28.2 to 51.1 mg GAE/mg dw of apple pomace and ORAC value at 200°C was about 5 times that at 100°C. An average increase of five times of antioxidant activity was reported using TEAC assay from 100 to 200°C, under subcritical water extraction of phytochemicals from plant sources [32]. ORAC values correlates positively with total phenolic content of the extracts because similar trends were observed and can be seen clearly by their surface response behavior as in Figures 4a and 4b. Both ORAC and total phenolic content are antioxidant capacity assays with different reaction mechanisms. Previous reports of convincing relationship between total phenolic content and antioxidant activities have been documented [35,43,44]. With increasing both the total phenolic content and the antioxidant activity at the high temperature resulted in diminishing amount of ordinary polyphenolic compounds in extracts. Therefore, the high antioxidant activity registered cannot be attributed to ordinary phenolic compounds alone at the higher temperatures. New polyphenolic compounds with much higher antioxidant activity could be formed which were not detected by the chromatographic protocol. Some researchers have linked the increased antioxidant activity at higher temperatures to the formation of new antioxidant compounds [9,10]. These new compounds may possess different chemical structures from their parent polyphenolic compounds and antioxidant capacities of polyphenolic compounds are affected by their chemical structure [45]. Protocatechuic aldehyde was identified in the extracts at 150°C and the UV spectra of standard (red marking) and those in samples (blue marking) are shown in Figure 5.
Protocatechuic aldehyde which is a degradation product of some hydrocinnamic acids was stable in the extract at 200°C and could contribute to antioxidant activity. Anti-inflammatory effects antioxidant activity of water soluble Protocatechuic aldehyde on Human Umbilical Vascular Endothelial Cells (HUVECS) have been documented. ORAC value of Protocateuchuic aldehyde was comparable to Caffeic acid and slightly higher than Chlorogenic acid.
Optimization of process and verification of model for total phenolic content under SWE
The transformed quadratic model was used to optimize extraction conditions with the objective to achieve maximum total phenolic content from apple pomace. The extraction conditions for the optimization were set using the numerical optimization option and the design expert produced series of solutions with varying desirability and the best solution with maximum desirability was selected. Subcritical water extractions were done in triplicate and total phenolic content determined and results shown in Table 8.
| Solid/solvent (%) | Extraction Temp (°C) | Extraction time (min) | Predicted TPC (mg GAE /g) | Actual TPC (mgGAE/g) |
|---|---|---|---|---|
| 1 | 200 | 30 | 53.76 | 49.86 ± 1.6 |
Table 8: Optimal conditions for extracting phenolic compounds with TPC content of predicted and experimental values.
Identification of polyphenolic compounds under subcritical water extracts
The extracts from the subcritical water extraction were analyzed by the HPLC-DAD method. The main polyphenolic compounds present between 100-150°C in the subcritical water extract were, Chrologenic acid, Phloridzin, Quercetin-3-galactoside, Quercetin-3-glucoside, Procyanidins B2 and Phloretin glycosides. Protocatechuic aldehyde was detected at 150°C and beyond. The identified polyphenolic compounds except Protocatechuic aldehyde were among polyphenolic compounds recovered from apple pomace using pressurized ethanol/ water as solvents, with other phenolic compounds like Caffeic acid, P-Coumaric acid, Catechins and Procyanidins, detected only at smaller concentrations [46-49]. Although the apple pomace residue used in the extraction were a blend including the red varieties of cider apples, anthocyanidins were not detected. Anthocyanidins are highly unstable and may oxidize before the start of extraction process [50-53]. Other peaks typically of polyphenolic nature by their UV- spectra were present. However, standards were not readily available to identify them. Hydroxymethylfurral (5-HMF), Furfural were detected as temperature increases from 100°C [54,55]. Chromatograms at 280 nm and 320 nm of the subcritical water extraction of the apple pomace are shown in Figures 6-8 and that of selected standard is shown in Figure 9.
Figure 7: Chromatogram of subcritical water extract for 4.5% solid-to-solvent ratio at 150°C for 30 minutes; 1-5HMF; 2-furfural, 3- Protocatechuic aldehyde; 4- Chlorogenic acid; 5-(isomer of Chlorogenic acid); 6-caffeic acid; 7-Quercetin-3- galactoside; 8-Quercetin-3-glucoside, 9 and 10 not identified and 11-Phlorodzin.
Polyphenolic compounds were successfully recovered from wet apple pomace under subcritical water extraction. Multivariate statistics procedure employed in the current studies demonstrated and predicted the behavior of temperature, solid to solvent ratio and resident time of extraction on overall recovery of polyphenolic compounds. Temperature, solid to solvent ratio and residence time influenced selectivity in the extraction of polyphenolic compounds from the wet apple pomace, which demonstrated high antioxidant activity by ORAC assay. Total phenolic content and antioxidant activity as determined by ORAC assay continued to increase with increasing temperature up to 200°C. The statistical methods employed predicted optimum phenolic content and antioxidant activity of apple pomace by response surface methodology at temperature 200°C using 1% solid to solvent ratio for 30 minutes’ residence time. Chlorogenic acid, Procyanidin B2, Phloridzin and Quercetin glycosides detected from 100°C to 150°C were no longer present at 200°C. Hence phenolic acids and flavonoids recovered below 150°C would have little contribution to total antioxidant activities at higher temperatures. For the first time, Protocatechuic aldehyde was identified only in the subcritical water extract and to date has not been reported in solvent extracts of cider apple pomace. Subcritical water mediated extraction of polyphenolic compounds was shown to be efficient due to the simultaneous hydrolysis and solubilization of the apple pomace than aqueous acetone and can be an alternative source of natural antioxidants for nutraceutical, pharmaceutical and cosmetic industries.
This current research was supported financially throughout by Ghana Education Trust Fund (GETFund). The authors wish to thank Universal Beverages Limited (UBL), a subsidiary company of Bulmers, UK who supplied the apple pomace sample.