Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2017) Volume 8, Issue 3
In this research, Loess Soil Nanoparticles (LSN), as a novel adsorbent nanocomposite was used for removal of green malachite (GM) dye from Aqueous Solutions by in a batch and fixed bed column. Firstly, LSN adsorption properties were investigated. The study investigates the effect of process parameters such as pH, adsorbent dosage, contact time and GM dye initial concentration. Next, GM dye was quantitatively evaluated by using the Langmuir, Freundlich isotherm and pseudo first and second order model. The adsorption data follow the adsorption equilibrium was described well by the Freundlich isotherm model. The results of SEM and AFM analysis show that particle size is less than 100 nm. Also, the BET analysis shows that the surface areas for LSN are 70 m2/g respectively. The results show that adsorption capacities and removal percentage of GM dye on LSN from wastewaters is about 80%. Consequently LSN is a superior adsorbent for wastewaters purification.
Keywords: Loess soil nanoparticles; Green malachite dye; Adsorption; Waste water treatment
Various colorants (dyes and pigments) are being applied in many industries for different coating applications. It is the inevitable reason for existence these materials in industrial wastewater. Colored wastewaters, especially organic ones, are wastes of different industries, such as paper, textile, leather, food, polymers, minerals and plastics [1]. These cause that treatment of water and wastewater, contaminated with colorants, are one of the main concerns of researchers in recent decades. In a real wastewater, there is a complex of different materials, such as colorants, polyacrylates, phosphonates, anti-coagulation factors, and so on. Most of these compounds are poisoning and it is necessary for ecological balances that these dangerous contaminants are being removed from treated wastewater completely. Therefore, the governments and different UN organizations have recently established many rules to prevent and standardize these materials in environment [2,3]. Different physicochemical decolorization processes have been developed to remove contaminants from industrial wastewater in recent years, such as reduction and precipitation [4], coagulation and flotation [5] membrane technologies and electrolysis [6,7], biological treatments [8], advanced oxidation processes [9], chemical and electrochemical techniques [10,11] and adsorption procedures [12-15]. Among all the treatments proposed, adsorption using sorbents is one of the most popular methods. It is now recognized as an effective, efficient and economic method for water decontamination applications and for separation to analytical purpose [16].
But most of these methods are expensive and certain economical foundation is necessary. Among physicochemical processes, adsorption technology has found many applications in water and wastewater treatment, as one of the most efficient and effective technologies [2,17]. Therefore, natural adsorbents have been used to reduce costs and the environmental side effects, such as diatomite [2], red mud [18], chitosan [19], orange skin [20], soy meal hull [21], almond skin [22], sawdust [23], zeolite [24] and clay [25]. These adsorbents have natural base and they are environmental friendly. It is possible to regenerate most of them or be applied in different products.
The nanometer material is a new functional material, which has attracted much attention due to its special properties. Most of atoms on the surface of the nanoparticles are unsaturated and can easily bind with other atoms. Nanoparticles have high adsorption capacity. Besides, the operation is simple, and the adsorption process rapid. So, there is a growing interest in the application of nanoparticles as adsorbents [26].
Nowadays, with helping new methods such as magnetization the efficiency of natural adsorbents to adsorption of pollutants from aqueous solution slightly decreased [27,28] rather natural adsorbent, but the adsorbent material can be easily separated from a mixture of particles using a simple magnet. So, in this research, removal of GM dye has been investigated from simulated textile wastewaters by LSN. Loess Soil is a natural and cheap adsorbent. So, Loess Soil Nano scale size of this adsorbent was prepared by mill (EQ-PC-12 model).
So, the objective of the present study is focused on the development of LSN for removal of GM dye. The dye selected in this study was malachite green because of their environmental significance. The effects of pH, adsorbent dosage, contact time and GM dye initial concentration were investigated. Since optimization of parameters, the characterization of isothermal adsorption and adsorption kinetics and also the SEM, AFM and BET analysis were studied in order to provide a new method and theoretical evidences for wastewater treatment.
Experimental Studies (Materials and Equipment)
Loess Soil Nanoparticles used in this investigation was obtained from NanoMineTech Company (a source in Iran). It can be prepared in any material construction store. Green Malachite (GM) dye was supplied from Ciba Company. Its molecular structure has been shown in Figure 1.
A laboratory balance (Sartorius-d=0.1 mg, max 120 g model) was used to weight samples. Some simple laboratory heater-stirrer systems were used to mix samples; UV/Visible spectrometer (One beam), high temperature oven 1100°C for drying (Cecil-CE2021-2000 series) was applied to measure the change on concentration of GM dye. In addition, various sieves with different meshes were used to categorize the adsorbent. A Centrifuge (Hettich EBA20, maximum whirl=6000 rpm) was applied to sediment and remove colloidal particles, and pH meter (Metrohm 713) was used to measure and adjusting the pH of simulated wastewaters. A mill (EQ-PC1-12) that crush particle by physical method, and a spray dryer (BUCHI B-191) for drying slurry particles, SEM (Scan Electron Microscope, LEO 1455VP), AFM (Atomic Force Microscope, Model SZMU-L5) and BET analysis was used to increase the knowledge about Loess Soil Nanoparticles microscopic structure and its real nature. Other chemicals, such as Sodium hydroxide and Chloridric acid were supplied from Merck Company, especially to adjust the pH of wastewater.
Adsorption procedure
The adsorption measurements were conducted by mixing various amounts of Loess Soil Nanoparticles (0.05 g) in stirrer containing a dye solution (6, 12, 20 ppm) of GM dye at temperature 25°C and pH=6 for 120 min to attain equilibrium condition. The mixing rate was high enough (>3000 rpm) to minimize the external mass transfer resistance. The changes in adsorption were determined at certain times (0, 10, 30, 60, 90, 120 min) during the removal process. After conducting adsorption experiment, the solution was separated from LSN by centrifugation at 4000 rpm for 5 min using a Hettich EBA20 centrifuge. The percentage adsorption of dye from aqueous solution was computed as follows
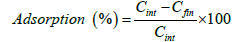 (1)
(1)
Where, Cint and Cfin are the initial and final dye concentrations, respectively. The dye concentrations in aqueous solution were determined using CECIL 2021 spectrophotometer corresponding to the maximum wavelength (λ max=619 nm ) of malachite green.
Effect of pH
The pH of the solution is an important factor that controls the adsorption process. The effect of pH on the green malachite adsorption by LSN was investigated in the pH of 4, 6, and 8. Figure 2 shows the malachite green adsorption percentage as a function of pH and contact time. As it is shown in Figure 2, dye adsorption fell with an increase in pH parameter. The maximum removal take place at pH=6.
Effect of loess soil nanoparticles dosage and contact time
The effect of the LSN dosage on the removal of GM dye which was based on the contact time was studied by changing at the adsorbent dosage in the range of 0.01, 0.03, 0.05, 0.07 g. The results are presented in Figure 3.
Based on Figure 4, removal trend of GM dye from 20 ppm simulated wastewater in pH=6 by 0.01 g, 0.03 g, 0.05 g and 0.07 g per 25 ml of solution was done. The dosage 20 ppm and 0.05 g LSN have the most effective adsorption value. Additionally, the adsorption yield increased with contact time and attained a maximum value at 120 min. Applying more adsorbent than the optimized dosage (0.05 g) has diminished the capacity of adsorption process. This phenomenon is due to a fluid mechanical problem. By raising the amount of adsorbent, coagulation of the particles would decrease the surface area of the adsorbent and would lessen the capacity of the LSN.
X- ray diffraction
The chemical constituent of the LSN as adsorbent for GM dye was calculated using X-Ray Diffraction (XRD) method. X-Ray diffraction (XRD) study using a Philips X-Ray Diffractometer Xunique 1140 was operated to characterize the minerals existing in LSN phase is the major phase at the Loess Soil Nanoparticles samples (Figure 5). It is obvious that the Quarts phase is the major phase.
Effect of GM dye initial concentration
The effect of GM Dye initial concentration on the adsorption process was investigated by changing the dye initial concentration at the range of 6, 12 and 20 ppm under optimized conditions (pH=6, contact time of 120 min, adsorbent dosage of 20 ppm and temperature of environment). The GM Dye adsorption efficiency was decreased by increasing the initial dye concentration. When GM Dye concentration increased from 6 to 20 ppm the adsorption percentage increased (Figure 4).
XRF analysis
Chemical composition of Loess Soil Nanoparticles sample was characterized by XRF, (Philips Diffractometer Xunique п) in the Central Laboratory of Amirkabir University of Technology, and the result are presented in Table 1. As can be seen in this table, based on XRF analysis, LSN sample is composed mainly of SiO2 and Al2O3
| Sample (%) | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | MgO | TiO2 | MnO |
|---|---|---|---|---|---|---|---|---|---|
| 57.32 | 18.91 | 10.42 | 0.96 | 1.08 | 2.38 | 2.70 | 1.103 | 0.147 | |
| P2O5 | S | L.O.I | Cl | Ba | Sr | Cu | Zn | Pb | |
| 0.203 | 0.002 | 4.45 | 53 | 320 | 177 | 35 | 158 | 24 | |
| Sample (ppm) | Ni | Cr | V | Ce | La | W | Zr | Y | Rb |
| 102 | 118 | 172 | 114 | 51 | 1 | 180 | 48 | 91 | |
| Co | As | U | Th | Mo | Ga | Nb | |||
| 5 | 4 | 2 | 5 | 3 | 15 | 11 |
Table 1: Results of XRF analysis, density and color of LSN samples.
Minerals under the microscope
Loess is an Aeolian sediment formed by the accumulation of windblown silt, typically in the 1–150 micrometer size range, twenty percent or less clay and the balance equal parts sand and silt that are loosely cemented by calcium carbonate. It is usually homogeneous and highly porous and is traversed by vertical capillaries that permit the sediment to fracture and form vertical bluffs. The Loess Consists of Quarts, Tourmaline, Pyrite and Calcite (Figure 6).
SEM analysis of loess soil nanoparticles
The images of the LSN surfaces were obtained using scanning electron microscope (SEM) instrument, model LEO 1455VP, illustrating raw Loess (Figure 7a) and Loess Soil Nanoparticles before (Figure 7b) and after GM dye adsorption (Figure 7c). This characteristic causes that LSN to be a proper adsorbent. Moreover, comparison of the Figures 7b and 7c show that the Nano surfaces have been treated and prepared to adsorb pollutants from the wastewater and the structure of the surface of LSN completely changed before and after process. The extra parts and porosity have been removed.
AFM and BET analysis of loess soil nanoparticles
Atomic force microscopy (AFM) is a powerful tool allowing a variety of surfaces to be imaged and characterized at the atomic level. According to Figure 8, LSN as a Nano adsorbent have a high porosity and this porosity as an effective factor have an important role at the GM dye adsorption. The results of BET analysis surface areas for LSN was 70 m2/g.
Adsorption isotherms
Adsorption isotherms describe how adsorbents interact with adsorbents. Adsorption isotherms demonstrate the relationships between equilibrium concentrations of adsorbate in the solid phase, q and in the liquid phase, C at constant temperature [22,29-31]. Adsorption isotherms are described in many mathematical forms. They are often obtained in the laboratory using batch test in which the equilibrium data are attempted by various isotherm models, such as Langmuir, Freundlich [32-34] isotherms. The Langmuir isotherm model suggests that the uptake of adsorbate occurs on the homogeneous surface by monolayer sorption without interaction between adsorbed molecules. The model assumes that the energies of adsorption on the surface are uniform and no migration of adsorbate happens on it. The linear form of Langmuir isotherm equation is represented by the following equation [35].
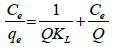 (2)
(2)
Where Ce is the equilibrium concentration of adsorbate (mg/L), qe is the amount of adsorbed at equilibrium (mg/g), Q (mg/g) and KL (L/mg) are Langmuir constants related to the adsorption capacity and energy, respectively. When qe is plotted against Ce, a straight line is obtained with slope of 1 Q adsorption of GM dye follows the Langmuir parameters.
The Freundlich equation has been widely used and is applicable to isothermal adsorption. This model is a special case for heterogeneous surface energies in the Langmuir equation. The energy term varies as a function of surface coverage, qe in this model. qe Strictly depends on the variations in heat of adsorption [36]. The Freundlich equation has the following general form [22,35].
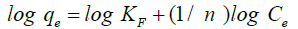 (3)
(3)
where, qe the amount of is adsorbed per until weight (mg/g adsorbent), Ce is the equilibrium concentration of adsorbate (mg/L), KF and n are the Freundlich constants.
The empirical parameters for Loess Soil Nanoparticles are given in Table 2. The fitting of experimental data in each isotherm model were examined by calculation of the correlation factor (R2). It was found that the Freundlich model better describes the adsorption than the Langmuir models according to correlation factor (R2=1.0). As can be seen from this table, the highest R2 values of Freundlich model shows that it is the most suitable equation to describe the adsorption equilibrium. According to this model, the Freundlich constants Kf and n were calculated 7.0345 and 2.987 for LSN. These are relatively uncommon but are often observed at low concentration ranges for compounds containing a polar functional group. Consequently, the adsorption of GM dye by LSN follows the Freundlich isotherm model.
|
|
Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| Adsorbent type | Q | KL | R22 | KF | n | R12 |
| Loess Soil Nanoparticles | 70 | 0.018 | 0.921 | 7.0345 | 2.987 | 0.989 |
Table 2: The isotherm coefficients for adsorption of GM dye on LSN.
Adsorption kinetics
It is essential to predict the rate at which dye is removed from aqueous solutions in order to design an appropriate treatment system based on adsorption process. Pseudo-first and pseudo-second order models have been applied to describe the adsorption kinetics of GM dye by LSN. The pseudo-first-order Kinetics model can be represented by the following Lagergren’s expression.
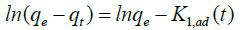 (4)
(4)
where, qe and qt are the amounts of dye adsorbed (mg/g) at equilibrium and at time t (min), respectively, and K1, ad is the pseudofirst- order rate constant (1/min). The rate of pseudo-second-order model depends on the amount of dye adsorbed on the surface of the adsorbent and its quantity at equilibrium condition [37]. Pseudo-firstorder model can be given as follows [38].
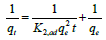 (5)
(5)
Where K2,ad is the rate constant of pseudo-second-order model (g/ mg min). The kinetics parameters of the pseudo- first-order and pseudosecond- order models of GM dye at different pH and temperatures are given in Tables 2 and 3.
| Pseudo-first-order | Pseudo-second-order | |||
|---|---|---|---|---|
| pH | K1,ad | R2 | K2,ad | R2 |
| 4 | 0.0038 | 0.5437 | 0.0006 | 0.4583 |
| 6 | 0.0081 | 0.702 | 0.002 | 0.892 |
| 8 | 0.0091 | 0.6426 | 0.0025 | 0.691 |
Table 3: Kinetics constants for GM obtained at pH=4, 6 and 8.
The results of this research show that the surface morphology of Loess Soil Nanoparticles has very important role on adsorption process in low bulk mass transfer velocity (rotational speed of stirrer). As a Novel low-price adsorbent, LSN was applied to remove GM dye from a simulated wastewater. Adsorption process of GM dye by LSN depends on different parameters, such as particles size, pH, adsorbent dosage and temperature. The maximum percentage of GM dye removal was about 80% which was obtained under normal temperature (25°C) and at pH=6. The results of fixed bed column show that 6, 8 and 10 mL/min flow rates the break through curve were saturated in 150, 300 and 450 min, respectively. Based on results the Freundlich model better describes the adsorption than the Langmuir models. Also, the adsorption kinetics of GM dye by LSN adsorbent can be well described by the pseudo-second-order reaction model at various pH values.
The authors are thankful to Nano Mine Tech Co. and College of Mining Engineering, University of Tehran, Tehran, Iran for supporting this research.