Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2016) Volume 7, Issue 2
Phosphorus is an essential macronutrient. A great portion of phosphorus from chemical fertilizers becomes insoluble and unavailable to plants because of its conversion into salts. Phosphate solubilizing microorganisms as a phosphorus biofertilizer improve soil fertility by solubilizing insoluble phosphate salts and increase crop production. This research aimed in isolation and characterization of phosphate solubilizing bacteria. Sixtythree soil samples collected from seven different parts of white lupin growing areas of Gojam, Ethiopia. Halo zone formation and plate screening method used for isolation. Based on halo zone formation; 152 phosphate solubilizing bacterial isolates obtained. Based on their solubilization index four isolates selected for subsequent experiments and characterization. The four selected isolates tested for their solubilization efficiency on solid media. Isolates HUPSB-35 and HUPSB-45 appeared maximum solubilization index (4.5 mm). Isolate HUPSB-57 was unique with its colony formation; salt tolerance (able to grow up to 10%) and utilization of wide range of carbon sources (utilize all the tested sugars). Isolate HUPSB-27 showed a wide range of pH and temperature preference (100% growth at 10, 15, 20, 25 and 30°C in all tested pH).
<Keywords: Biofertilizer; Isolates; Phosphorous; PSB
Phosphorus is one of the least available and the least mobile mineral nutrients in soil for plant growth. However, it is vital for plants and they absorb only inorganic form of phosphorous but the level of inorganic phosphorus is very low in soil because most of the phosphorous is present as insoluble forms [1,2]. Unlike nitrogen, there is no large atmospheric source that can be made biologically available to plants [1]. P chemical fertilizer is widely applied in agricultural production [2]. However, a large proportion of these fertilizers are also converted to insoluble form leads to low-fertilizer efficiency where the volume of phosphatic fertilizers as low as 15-20% are only utilized by plants due to fixation of P in acidic and alkaline soils [3].
Phosphorus deficiency is the most important problem of Ethiopian soils and more than 70-75% of highland soils are characterized by phosphorus deficiency [4]. Around 70% of Ethiopian vertisols have available phosphorus below 5 ppm, which is very low for supporting good plant growth; and, fixation in vertisols is related more to calcium, than Al3+ and Fe3+ [5]. Yield is usually low under traditional farming system because of poor cultural practices that is compounded with poor soil fertility. Though chemical fertilizers play a role in agricultural sector, the ever-increasing prices of chemical fertilizers have made them unfavorable for most farmers. Consequently, farmers are applying fertilizers below the recommended rates with yields lower than the potential of their cultivated crops. Thus, there is an urgent need to search for supplementary and cheaper source of nutrients, particularly those of nitrogen and phosphorus because the two nutrients often limit crop production.
One of the cheap sources for improving phosphorus nutrition is manipulation of phosphate solubilizing microbes (PSM) alone or together with cheap rock phosphate [6]. Different studies showed that selected microorganisms are found to be promising as important components of biofertilizer production towards the efforts of integrated soil fertility management [7]. Phosphate solubilizing microorganisms (PSMs) or phosphate solubilizing bacteria (PSB) can convert insoluble phosphates into soluble forms [8]. Population in the soil of PSMs varies from soil to soil and ranges from less than 102 colony forming units (cfu) g-1 of soil to 3 × 106 cfu g-1 of soil [9] reviewed that the most commonly found phosphate solubilizing bacteria (PSB) were belonging to genus Pseudomonas, Bacillus and some of other bacteria such as: Mycobacterium, Micrococcus, Flavobacterium, Achromobacter sp. of these genera, Pseudomonas sp. is the most efficient phosphate solubilizer.
In Ethiopia, the hitherto researches on the manipulation of microorganisms for soil fertility management have concentrated on understanding and improving nitrogen fixing microorganisms. However, previous researches also indicated that phosphate solubilizing microbes (especially bacteria) are very important in response to phosphorus deficiency in soil [4,10] evaluated the effect of co-inoculation of Bradyrhizobium japonicum and phosphate solubilizing Pseudomonas spp on soya bean, and got a promising result in Assossa area. Some of the root nodule bacteria were also effective phosphate solubilizers, there is no extensive research undertaken on the status of PSB in lupin growing areas. Hence, the objective was to isolate and characterize efficient phosphate solubilizing bacteria from white lupin rhizosphere by evaluating their efficiency on solid media.
Study area
The sampling areas covered 63 sampling sites from seven different parts of Gojam, Amhara Region, Ethiopia. Areas include: Dangila, Debre-Marekose, Rob-Gebeya, Dembecha, Kossober, Merawi and Tilili that are located between 1110’59.880”N, 3953’60.000” E and known for lupin production. White lupin was selected for rhizosphere soil sample collection due to its ability to grow on different soil types, area and high soil fertility maintenance capacity. Farmers in the study area cultivate lupin with intercropping basically for fertility maintenance other than crop production. The research conducted at Holeta Agricultural Research Center.
Study design
A cross sectional (for soil sampling) and laboratory based (for experiment) design were followed. Soil samples were randomly excavated in triplicates from a depth of 0-30 cm under the rhizosphere of lupin plants, sub-sampled and separately collected in sterile plastic bags from Jan 2011- Feb 2012. Soils were taken to Chemistry Laboratory for analysis and to Holeta Agricultural Research Center for isolation of phosphate solubilizing bacteria.
Isolation of phosphate solubilizing bacteria from soil
Each soil sample was grind in to smaller parts with mortar and pestel and sieved with 2 mm mesh size. 10 gm of fine soil mixed thoroughly with 90 ml of sterilized distilled water for about 15 min. From the suspension, one ml transferred to 9 ml of sterilized 0.85% of normal saline solution for serial dilution up to 10-3(v/v). From the last dilution (10-3) [11], 100 μl was spread on plates of Pikovskaya’s agar medium (PVK-medium). Pikovskaya’s agar medium composition; Glucose, 10 gm/l; Tricalcium phosphate, 5 gm/l; Ammonium sulphate, 0.5 gm/l; Potassium chloride, 0.2 gm/l; Magnesium sulphate, 0.1 gm/l; Manganous sulphate, Traces (0.0002); Ferrous sulphate, Traces (0.0002); Yeast extract, 0.5 gm/l; Agar, 18 gm/l; pH, 7.0 and autoclave 121°C for 15 min. Plates were incubated at 30°C for 5-8 days. Bacterial colonies showing clear zone were then sub-cultured, purified, and preserved at 4°C in the Microbial Biotechnology Lab. of Holeta Agricultural Research Center. Isolates designated as; HUPSB followed with serial numbers.
Screening for phosphate-solubilization in petri plates
Estimation of phosphate solublization ability of isolates was undertaken using plate screening methods. Hundred micro-liters of pure isolates from 48 hrs broth culture spread evenly under aseptic conditions on Pikovskaya’s agar [12], and incubated at 30°C. The halo and colony diameter measured at 2, 4, 6 and 8 days of incubation. To calculate their solubilzation index [13].
SI=(HZD+CD)/CD
Where; SI=Solubilization index, HZD=Halo zone diameter, CD=Colony diameter.
Characterization of isolates
Based on their solubilization index 4 bacterial isolates, 3 rhizosphere bacteria with high solubilization index (HUPSB- 27, HUPSB-35, HUPSB-45) and one isolate with low index (HUPSB-57) were selected for biochemical and physiological characterization. Gram staining, motility, cultural characteristics (colony color, texture and shape), biochemical (growth on different carbon sources), pH and temperature tests of PSB isolates carried out according to the procedure of Ref. [14].
Mean generation time: The standard curve of isolates was plotted by measuring Optical Density at wavelength of 600 nanometer and relating with colony forming units (CFUs) obtained from plates. Mathematically mean generation time (g) calculated according to Ref. [15],
g=t/n,
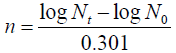
Where, n=The number of generations in time t
N0=The initial population number
Nt=The population at time t
Utilization of other substrates by isolates: Molasses, urea, and Egyptian rock phosphate (ERP) were evaluated as alternative sources for carbon, nitrogen, and tricalcium phosphate (TCP) respectively incorporated in PVK agar. Loopful of 48 hrs grown culture of each isolate was streaked and incubated at 30°C for 5 days.
pH vs. temperature test: Isolates were tested for their ability to grow at different pH in PVK liquid medium adjusted to pH 4, 5, 6, 7, 8, 9, and 10 in relation with different temperature of 5, 10, 15, 20, 25, 30, 35, 40 and 45°C [16].
Salt tolerance test: Isolates were grown on PVK medium with different concentrations of salt (NaCl). The tested concentrations were 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10%. The result was recorded after 5 days incubation at 30°C at neutral pH (7.0).
Data analysis
Upon eight days incubation isolate’s colony diameter and halozone formation were measured. These used to calculate SI for each isolate and set their average values. Data from OD measurements and CFU counts for selected isolates were analyzed using SAS 9.1.3 version with Fisher’s LSD (significance set at P ≤ 0.05) and presented in table.
Isolation of PSB
A total of 152 PSB isolates were obtained; some of them indicated in Table 1 including colony diameter, halo zone diameter and solubilization index (SI) upon 2- 8 days of incubation. The largest halo zone (15 mm) recorded from isolates HUPSB-57Mer1 followed by HUPSB-17D/M1 (10 mm); whereas the smallest halo zone (3 mm) was measured from isolate HUPSB-34T1. Likewise, from tested isolates, the highest solubilization index (SI) was shown by isolates HUPSB-35 and HUPSB-45 (4.5 mm) and the smallest SI from isolate HUPSB-57 (2.2 mm). In general, the average SI of isolates found to be 2.8 (Table 1). More over different isolates could be isolated from different parts of the world with different solubilization efficiency. Variation in their SI could possibly the deference in; isolates type, natural environment, physicochemical properties of soil, soil management and agricultural practices. In addition, the importance of isolation from local environment is to avoid competition with indigenous microbes.
| Isolates | Day 2 | Day 4 | Day 6 | Day 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diameter (mm) | Diameter (mm) | Diameter (mm) | Diameter (mm) | |||||||||
| C | H | SI | C | H | SI | C | H | SI | C | H | SI | |
| HUPSB-3-RG1 | 2 | 3 | 2.5 | 3 | 4 | 2.3 | 5 | 7 | 2.4 | |||
| HUPSB-5-RG2 | 1 | 2 | 3 | 2 | 5 | 3.5 | 3 | 6 | 3 | |||
| HUPSB-5-RG8 | 1 | 2 | 3 | 2 | 4 | 3 | 2 | 4 | 3 | |||
| HUPSB-5-RG10 | 1 | 2 | 3 | 3 | 5 | 2.7 | 4 | 7 | 2.8 | |||
| HUPSB-5-RG11 | 1 | 2 | 3 | 3 | 5 | 2.7 | 4 | 6 | 2.5 | |||
| HUPSB-6-RG3 | 2 | 2 | 2 | 2 | 4 | 3 | 3 | 5 | 2.7 | |||
| HUPSB-7-RG2 | 1 | 2 | 3 | 2 | 3 | 2.5 | 2 | 4 | 3 | |||
| HUPSB-8-RGI | 2 | 3 | 2.5 | 3 | 6 | 3 | 4 | 7 | 2.8 | |||
| HUPSB-9-RG1 | 1 | 1 | 2 | 1 | 2 | 3 | 3 | 4 | 2.3 | 4 | 6 | 2.5 |
| HUPSB-13-D/M2 | 4 | 6 | 2.5 | 4 | 6 | 2.5 | 4 | 6 | 2.5 | |||
| HUPSB-13-D/MI | 3 | 5 | 2.7 | 3 | 7 | 3.3 | 3 | 7 | 3.3 | |||
| HUPSB-14-DEM1 | 1 | 2 | 3 | 3 | 6 | 3 | 4 | 7 | 2.8 | |||
| HUPSB-15-D/MI | 2 | 3 | 2.5 | 3 | 5 | 2.7 | 4 | 6 | 2.5 | |||
| HUPSB-16-D/MII | 1 | 2 | 3 | 2 | 4 | 3 | 3 | 5 | 2.7 | |||
| HUPSB-17-D/M1 | 3 | 6 | 3 | 4 | 8 | 3 | 6 | 10 | 2.7 | |||
| HUPSB-18-D/M1 | 1 | 1 | 2 | 2 | 3 | 2.5 | 3 | 6 | 3 | |||
| HUPSB-19-DEM3 | 1 | 2 | 3 | 2 | 4 | 3 | 2 | 4 | 3 | |||
| HUPSB-24-DEM I | 2 | 3 | 2.5 | 2 | 3 | 2.5 | 3 | 5 | 2.7 | |||
| HUPSB-26-DEM I | 2 | 4 | 3 | 3 | 6 | 3 | 3 | 7 | 3.3 | |||
| HUPSB-27-DEM1 | 1 | 1 | 2 | 1.5 | 3 | 3 | 2 | 5 | 3.5 | 2 | 5 | 3.5 |
| HUPSB-27-DEM2 | 1 | 1 | 2 | 1 | 3 | 4 | 2 | 5 | 3.5 | 3 | 6 | 3 |
| HUPSB-30-T1 | 1 | 1 | 2 | 2 | 4 | 3 | 3 | 5 | 2.7 | 5 | 8 | 2.6 |
| HUPSB-30-T2 | 1 | 2 | 3 | 2 | 5 | 3.5 | 3 | 6 | 3 | |||
| HUPSB-31-T21 | 2 | 2 | 2 | 2 | 3 | 2.5 | 3 | 7 | 3.3 | |||
| HUPSB-32-T1 | 1 | 2 | 3 | 1 | 2 | 3 | 2 | 4 | 3 | |||
| HUPSB-33-T1 | 1 | 1 | 2 | 2 | 5 | 3.5 | 3 | 5 | 2.7 | |||
| HUPSB-34-T1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 2.5 | |||
| HUPSB-34-T2 | 2 | 2 | 2 | 2 | 3 | 2.5 | 3 | 5 | 2.7 | |||
| HUPSB-35-T1 | 1 | 2 | 3 | 2 | 5 | 3.5 | 2 | 7 | 4.5 | |||
| HUPSB-36-T2 | 1 | 1 | 2 | 2 | 4 | 3 | 2 | 4 | 3 | 2 | 4 | 3 |
| HUPSB-39-K1 | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 4 | 2.3 | 4 | 6 | 2.5 |
| HUPSB-40-K1 | 1 | 1 | 2 | 2 | 3 | 2.5 | 3 | 5 | 2.7 | 4 | 6 | 2.5 |
| HUPSB-40-K2 | 1 | 1 | 2 | 2 | 4 | 3 | 2 | 5 | 3.5 | 3 | 5 | 2.7 |
| HUPSB-44-K1 | 3 | 5 | 2.7 | 4 | 7 | 2.8 | 6 | 9 | 2.5 | |||
| HUPSB-44-K2 | 2 | 4 | 3 | 3 | 5 | 2.7 | 3 | 7 | 3.3 | |||
| HUPSB-44-K3 | 1 | 1 | 2 | 2 | 3 | 2.5 | 4 | 6 | 2.5 | |||
| HUPSB-45-K2 | 1 | 1.5 | 2.5 | 1 | 2 | 3 | 2 | 5 | 3.5 | 2 | 7 | 4.5 |
| HUPSB-48-DAN1 | 1 | 1 | 2 | 2 | 4 | 3 | 3 | 5 | 2.7 | 4 | 6 | 2.5 |
| HUPSB-48-DAN3 | 1 | 1 | 2 | 2 | 3 | 3 | 2 | 5 | 3.5 | 3 | 5 | 2.7 |
| HUPSB-50-DAN1 | 1 | 3 | 4 | 2 | 3 | 2.5 | 3 | 4 | 2.3 | |||
| HUPSB-50-DAN2 | 1 | 2 | 3 | 2 | 3 | 2.5 | 2 | 4 | 3 | |||
| HUPSB-52-DAN1 | 1 | 1 | 2 | 2 | 4 | 3 | 2 | 4 | 3 | |||
| HUPSB-55-MER4 | 1 | 3 | 4 | 3 | 5 | 2.7 | 4 | 7 | 2.8 | |||
| HUPSB-57-MER2 | 3 | 3 | 2 | 4 | 5 | 2.3 | 5 | 6 | 2.2 | |||
| HUPSB-57-MER4 | 2 | 4 | 3 | 3 | 5 | 2.7 | 3 | 6 | 3 | |||
| HUPSB-57-MER5 | 4 | 4 | 2 | 4 | 5 | 2.3 | 4 | 5 | 2.3 | |||
| HUPSB-57-MER1 | 2 | 2 | 2 | 7 | 9 | 2.3 | 8 | 10 | 2.5 | 9 | 15 | 2.7 |
| HUPSB-57-MERIV | 1 | 1 | 2 | 2 | 4 | 3 | 3 | 6 | 3 | 4 | 7 | 2.8 |
| HUPSB-60-MER1 | 2 | 4 | 3 | 3 | 5 | 2.7 | 2 | 4 | 3 | |||
| HUPSB-61-MER1 | 2 | 2 | 2 | 2 | 4 | 3 | 4 | 6 | 2.5 | |||
| Average | 1.7 | 2.9 | 2.7 | 2.8 | 4.9 | 2.7 | 3.7 | 6.2 | 2.8 | |||
NB: DAN: Dangila; D/M: Debre Markose; DEM: Dembecha; K: Kosober; MER: Merawi; RG: Robgebeya; T: Tilili; C: Colony; H: Halo zone; SI: Solubilization index; HUPSB: Haramaya University Phosphate Solubilizing bacteria.
Table 1: Measurement of Halo and Colony Diameters of Isolates upon 8 Days Incubation.
Selection and characterization of PSB
Based on SI four isolates were selected for further tests and experimental activities. From the selected four isolates, all except HUPSB-35 formed halo zone starting from day two, and increased in halo zone size from 1-2 mm to 5-15 mm upon 8 days of incubation. They also displayed Solubilization Index (SI) of 2-2.5 up to 2.7-4.5. The most effective isolate, HUPSB-45 showed SI of 2.5 at day two and 4.5 upon 8 days incubation. Isolate HUPSB-35, which failed to show any solubilization zone on 2nd day of incubation showed a SI of 4.5 similar to isolate HUPSB-45. On the contrary, isolate HUPSB-57, which showed fastest solubilization at day two with larger SI of two did not show considerable change in SI (2.7) upon 8 days of incubation (Table 1). The data showed that there is steady increase in solubilization as incubation time increases and also HUPSB-27, HUPSB-35, and HUPSB-45 showed similar pattern of solubilization within 4-6 days.
Cultural and growth characteristics of isolates
Four selected isolates were taxonomically characterized based on their cultural, morphological, and growth characteristics (Table 2). Accordingly, all isolates were gram negative, rod shaped motile bacteria with round colony margin on solid media, except isolate HUPSB-57 (round irregular margin). With regard to colony colour, isolates HUPSB-27 and HUPSB-57 appeared to be white, whereas isolates HUPSB-35 and HUPSB-45 showed a yellow colour. Although isolates HUPSB-27 and HUPSB-57 were white, they differ in their transparency; in that; the latter was opaque and mucoid whereas the former was transparent and spreading. The isolates mean generation time range from 4.1 to 5.8 hrs and their OD values in relation to CFUs presented in Table 3. Relatively HUPSB-35 showed shorter doubling time (4.1 hrs) while HUPSB-27 had longer doubling time (5.8 hrs).
| Isolates | Colony color | Colony shape | Gram stain and cell shape | Motility | Mean Generation time (hrs) |
|---|---|---|---|---|---|
| HUPSB -27 | White | Round, RM | - R | + | 5.8 |
| HUPSB -35 | Yellow | Round, RM | - R | + | 4.1 |
| HUPSB -45 | Yellow | Round, RM | - R | + | 4.7 |
| HUPSB -57 | White | Round, IM | - R | + | 5.1 |
NB: RM: Regular margin; IM: Irregular margin; R: Rod shape; ‘-‘ gram negative; ‘+’ motile.
Table 2: Colony color, shape, gram stain, generation time and motility of the PSB isolates.
| Isolates | CFU (10-7) | OD | Incubation Time (hrs) |
|---|---|---|---|
| HUPSB-27-DEM1 | 0.3333d | 0.008000a | 0 |
| HUPSB-35-T1 | 1.0000c | 0.009333a | |
| HUPSB-45-K2 | 2.6667b | 0.013000a | |
| HUPSB-57-MER1 | 4.3000a | 0.014333a | |
| LSD | 0.2728 | 0.0071 | |
| CV | 6.982410 | 33.70624 | |
| Mean | 2.075000 | 0.011167 | |
| HUPSB-27-DEM1 | 7.6667b | 0.55633a | 6 |
| HUPSB-35-T1 | 2.7000c | 0.07367c | |
| HUPSB-45-K2 | 4.3000c | 0.05467c | |
| HUPSB-57-MER1 | 20.3333a | 0.40333b | |
| LSD | 1.9896 | 0.0637 | |
| CV | 12.07685 | 12.44084 | |
| Mean | 8.750000 | 0.272000 | |
| HUPSB-27-DEM1 | 53.6667a | 0.85333a | 12 |
| HUPSB-35-T1 | 7.0000d | 0.43533c | |
| HUPSB-45-K2 | 25.3333c | 0.51533b | |
| HUPSB-57-MER1 | 40.3333b | 0.82100a | |
| LSD | 1.0775 | 0.0647 | |
| CV | 1.811956 | 5.237784 | |
| Mean | 31.58333 | 0.656250 | |
| HUPSB-27-DEM1 | 73.333b | 1.48667a | 18 |
| HUPSB-35-T1 | 53.333d | 1.35533a | |
| HUPSB-45-K2 | 60.667c | 1.11000b | |
| HUPSB-57-MER1 | 103.000a | 1.40000a | |
| LSD | 4.7883 | 0.1897 | |
| CV | 3.503735 | 7.531417 | |
| Mean | 72.58333 | 1.338000 | |
| HUPSB-27-DEM1 | 106.000b | 1.57133a | 24 |
| HUPSB-35-T1 | 88.333c | 1.66667a | |
| HUPSB-45-K2 | 87.667c | 1.25333b | |
| HUPSB-57-MER1 | 151.000a | 1.57333a | |
| LSD | 3.957 | 0.1223 | |
| CV | 1.941420 | 4.284632 | |
| Mean | 108.2500 | 1.516167 | |
| HUPSB-27-DEM1 | 132.333d | 2.18533ba | 36 |
| HUPSB-35-T1 | 185.000b | 2.28767a | |
| HUPSB-45-K2 | 151.000c | 1.90700c | |
| HUPSB-57-MER1 | 203.333a | 2.14833b | |
| LSD | 7.4525 | 0.1138 | |
| CV | 2.357189 | 2.834303 | |
| Mean | 167.9167 | 2.132083 | |
| HUPSB-27-DEM1 | 147.000d | 2.41000a | 48 |
| HUPSB-35-T1 | 197.000b | 2.47767a | |
| HUPSB-45-K2 | 176.000c | 2.16333b | |
| HUPSB-57-MER1 | 219.333a | 2.33467a | |
| LSD | 5.1849 | 0.1464 | |
| CV | 1.489875 | 3.313546 | |
| Mean | 184.8333 | 2.346417 |
Table 3: OD and CFU value of the four selected isolates.
Sugar utilization of psb isolates
BTB (bromothyml blue) indicator used to identify sugar utilization. Isolate HUPSB-57 was the most efficient that utilized all of the given carbon sources whereas isolates HUPSB-45 and HUPSB-27 utilized fewer sugars (Table 4). Glucose, maltose and sucrose utilized by all of the isolates whereas lactose was utilized only by HUPSB-57.
| Isolates | Glu | Gala | Mal | Mani | Ara | Suc | Xyl | Fru | Lac | Dex |
|---|---|---|---|---|---|---|---|---|---|---|
| HUPSB-27 | + | - | + | + | + | + | - | + | - | - |
| HUPSB-35 | + | + | + | + | - | + | + | - | - | + |
| HUPSB-45 | + | + | + | - | - | + | + | - | - | + |
| HUPSB-57 | + | + | + | + | + | + | + | + | + | + |
NB: Glu: Glucose; Gala: Galactose; Mal: Maltose; Mani: Manitole; Ara: Arabinose; Suc: Sucrose; Xyl: Xylose; Fru: Fructose; Lac: Lactose; Dex: Dextrose; ‘+’ presence and ‘-‘ absence of growth.
Table 4: Sugar test of PSB isolates.
Growth of isolates on different substituted media
All the experimental isolates were able to grow on the substituted media of PVK: molasses agar, urea agar and PVK with ERP.
Growth of isolates at different pH and temperature
Isolates showed different response of growth in combinations of different medium pH (pH 4-10) and incubation temperature (5°C-45°C) (Table 5). No isolate was able to grow in any combination of pH and temperature of 45°C (Table 4). All isolates were found to grow at temperature of 10°C-25°C, at all tested pH values, except isolate HUPSB-57 that failed to grow under pH 10 and at 25°C. The most resilient isolate was HUPSB-27 that was able to grow at 30°C with all pH adjustments of the media (pH 4-10), and at 35°C and 40°C at pH 6 and pH 7 (Table 4). Isolates HUPSB-35 and HUPSB-45 showed similar pattern of growth at 30°C with pH 4-8, and 40°C at pH 7. In general, isolate HUPSB-27 performed better in different pH and temperature stress factors, whereas the two isolates (HUPSB-35 and HUPSB-45) showed similar pattern of tolerance. Isolate HUPSB-57 was relatively the most sensitive isolate to grow at different combinations of pH and incubation temperature.
| Isolate | pH | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| HUPSB-27 | - | + | + | + | + | + | + | 5 |
| + | + | + | + | + | + | + | 10 | |
| + | + | + | + | + | + | + | 15 | |
| + | + | + | + | + | + | + | 20 | |
| + | + | + | + | + | + | + | 25 | |
| + | + | + | + | + | + | + | 30 | |
| - | - | + | + | - | - | - | 35 | |
| - | - | + | + | - | - | - | 40 | |
| - | - | - | - | - | - | - | 45 | |
| HUPSB-35 | - | + | + | + | - | - | - | 5 |
| + | + | + | + | + | + | + | 10 | |
| + | + | + | + | + | + | + | 15 | |
| + | + | + | + | + | + | + | 20 | |
| + | + | + | + | + | + | + | 25 | |
| + | + | + | + | + | - | - | 30 | |
| - | - | - | + | - | - | - | 35 | |
| - | - | - | + | - | - | - | 40 | |
| - | - | - | - | - | - | - | 45 | |
| HUPSB- 45 | - | + | + | + | - | - | - | 5 |
| + | + | + | + | + | + | + | 10 | |
| + | + | + | + | + | + | + | 15 | |
| + | + | + | + | + | + | + | 20 | |
| + | + | + | + | + | + | + | 25 | |
| + | + | + | + | + | - | - | 30 | |
| - | - | - | + | - | - | - | 35 | |
| - | - | - | + | - | - | - | 40 | |
| - | - | - | - | - | - | - | 45 | |
| HUPSB-57 | - | + | + | + | - | - | - | 5 |
| + | + | + | + | + | + | + | 10 | |
| + | + | + | + | + | + | + | 15 | |
| + | + | + | + | + | + | + | 20 | |
| + | + | + | + | + | + | - | 25 | |
| + | + | + | + | + | - | - | 30 | |
| - | - | - | + | - | - | - | 35 | |
| - | - | - | + | - | - | - | 40 | |
| - | - | - | - | - | - | - | 45 | |
NB: ‘+’ presence and ‘-‘ absence of growth.
Table 5: Growth of isolate HUPSB-27, HUPSB-35, HUPSB-45 and HUPSB-57 at different pH and temperature.
Growth of isolates at different salt concentration
Salt tolerance of isolates was tested at different salt concentrations. All isolates were found to grow up to 2% salt concentration (0.5%, 1% and 2%) (Table 6). The most sensitive isolates were HUPSB-27 and HUPSB-35, that able to grow only up to 2% salt concentration. However, isolate HUPSB-57 showed a broad growth range and was able to grow in all tested salt concentrations (0.5-10%), followed by isolates HUPSB-45 grown up to 5% salt concentration.
| Isolates | [salt]% | ||||||||||
| 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| HUPSB-27 | + | + | + | - | - | - | - | - | - | - | - |
| HUPSB-35 | + | + | + | - | - | - | - | - | - | - | - |
| HUPSB-45 | + | + | + | + | + | + | - | - | - | - | - |
| HUPSB-57 | + | + | + | + | + | + | + | + | + | + | + |
Table 6: Growth at different salt concentration.
In general, isolates showed different cultural, morphological, growth, and eco-physiological characteristics. Isolates HUBSP-35 and HUBSP-45 were similar as they imparted yellow color on growth medium, the same pattern of SI of 4.5 after 8 days of incubation; temperature and pH tolerance, they differed in mannitol utilization, and salt tolerance. It can be argued that these four isolates can be tentatively grouped into genus (Pseudomonas) under different species. Isolates efficiency and symbiotic effectiveness were evaluated on soil under greenhouse condition. They showed significance difference over control in shoot height, shoot fresh and dry weight of white lupin as well as variation in total P content of white lupin and rhizosphere soil (data not shown).
Formation of clear zone is an indication of inorganic P-solubilization by isolates and measurement of SI on a solid medium is a very important and reliable tool for a preliminary screening of phosphate solubilizing microorganisms [17-19]. In Ethiopia, Keneni et al. reported that the largest clear zone diameter of 4.5 mm from three different PSB isolates (isolated from Mehalmeda) and Esubalew, reported the maximum SI of 1.8 by some isolates from Gojam soil.
All the tested isolates belong to fast growing bacteria according to Somasegaren and Hoben, who stated that fast growers would develop pronounced turbidity in liquid media within 2-3 days. Similarly, Esubalew, discussed white lupin nodulating and phosphate-solubilizing isolates displayed different doubling time ranging from 2-5. 3 hrs. The difference in all above cultural and growth characteristics may be due to genetic variation among isolates. Baon and Prescott, reported that bacteria growing on solid surfaces such as agar could form quite complex and complex colony shapes (or variation in colony shapes) [15,18].
On testing utilization of carbohydrates by white lupin nodulating bacteria including phosphate solubilizing and nodulating bacteria, Esubalew, stated that 95% of isolates grew in the presence of glucose. Poonam and Ghosh, also reported all of their isolates produced acid from glucose but only two out of five isolates were able produce acid from lactose. Nautiyal argued that the most efficient strain is the one that is capable of utilizing a wide range of carbon and nitrogen sources.
Experimental isolates were unable to grow after 80°C (data not shown). Similarly, Poonam and Ghosh, reported none of the isolates able to grow at high temperature (52°C). Prescott, discussed that cardinal temperatures for a particular species are not rigidly fixed but often depend to some extent on other environmental factors such as pH and available nutrients and also it varies between microorganisms.
The most sensitive isolates for salt tolerance were HUPSB-27 and HUPSB-35, which were able to grow at only 2% salt concentration. Similarly, Esubalew, reported that phosphate solubilising root nodule bacteria from lupin were able to grow at 2%. However, isolate HUPSB-57 was able to grow 0.5- 10%, which was similar to the work of; Poonam and Ghosh, who found that isolates could tolerate up to 10%; [20], where strain solubilize P at 10% and Zhu who found an isolate that could tolerate high salt concentration (20%).
In order to apply PSM/PSB as a biofertilizers, there need to be isolated and characterized. In this study, different phosphate solubilizing bacteria were isolated from different places with different solubilization efficiency and with different characters. The selected isolates were effective in solubilization of insoluble phosphorus on solid media. Phosphorous solubilization increased along the incubation days increased. This is true for plate screening (halo zone increased with increasing of colony diameter and resulted in SI increased).
First, we would like to thank Hawassa University, Haramaya University and the MoE for granting study opportunity, financial support and sponsorship for the study. Our great thanks go to Holeta Agricultural Research Center especially DSWRM, the technical assistants of Microbial Biotech. and Chemistry laboratories.