
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2023)Volume 13, Issue 2
Background: Refractory no-reflow syndrome can still occur, negating many of the benefits of restoring culprit vessel patency, and is associated with a worse in-hospital and long-term prognosis. Epinephrine may at lower doses exert potent beta receptor agonist properties that mediate coronary vasodilatation. The trial aims to estimate the efficacy and safety of the administration of intracoronary adrenalin, verapamil, as well as their combination versus standard treatment in patients with STEMI and refractory coronary no-reflow.
Methods/Design: Consecutive patients with STEMI and refractory no-reflow syndrome will be randomized into 4 groups: Standard therapy only, intracoronary administration of epinephrine, verapamil, epinephrine+verapamil. All patients will undergo an assessment of epicardial blood flow using TIMI score, peak troponin level, ST segment dynamics, echocardiography, MRI, dynamic SPECT.
Discussion: Based on the pharmacodynamic effects of epinephrine and verapamil, their combination is expected to have a more potent vasodilating effect. The study protocol follows the Declaration of Helsinki and has been approved by the ethics committee of the authors’ institution on October 14, 2020. The study complies with the national standard of good clinical practice (RF GOST P52379-2005 form April 1, 2006) and with the international guidelines for good clinical practice.
Conclusion: Investigators or investigator-authorized officers will be responsible for explaining the objective, content, study procedures, benefits, and risks of participating in the clinical trial to each patient or the patient's legal representative. Written informed consent will be obtained from all patients before the start of the trial.
ST elevation; Myocardial infarction; Percutaneous coronary intervention; No-reflow phenomenon
Primary Percutaneous Coronary Intervention (PPCI) is the preferred reperfusion strategy for treating acute ST-Segment Elevation Myocardial Infarction (STEMI). The main goals are to restore epicardial infarct-related artery patency and to achieve microvascular reperfusion as early as possible. No-reflow is the term used to describe inadequate myocardial perfusion of a given coronary segment without angiographic evidence of persistent mechanical obstruction of epicardial vessels. It refers to the high resistance of microvascular blood flow encountered during opening of the infarct-related coronary artery. Despite optimal evidence-based PPCI, myocardial no-reflow can still occur, negating many of the benefits of restoring culprit vessel patency and is associated with a worse in-hospital and long-term prognosis [1].
According to clinical guidelines, nitrates, adenosine, platelet IIb/ IIIa receptor inhibitors and thromboextraction can be used to prevent and treat this complication. These methods have demonstrated the ability to improve coronary blood flow in experiment and small clinical trials, however, limiting the zone of myocardial necrosis and improving disease outcomes have not been achieved [2]. The search for new methods of influencing the pathogenetic links of this complication is urgent. One of the main potentially reversible factors in the pathogenesis of the noreflow phenomenon, along with microvascular obstruction, is microvascular arteriolar spasm. Thus, this problem of emergency cardiology remains relevant and requires further research, new methods of prevention and treatment [3].
Aside from exerting at higher doses beta-1 agonist properties that increase inotropic and chronotropic stimulation of the myocardium, epinephrine may at lower doses exert potent beta receptor agonist properties that mediate coronary vasodilatation. In 2002, a study was conducted, in which 29 patients with noreflow phenomenon were injected with intracoronary adrenaline, which led to a significant improvement in coronary blood flow and the achievement of TIMI-3 in 69% of cases 11 [4]. The restore study included 30 STEMI patients who developed a refractory no-reflow phenomenon during primary PCI. Patients were randomized to either intracoronary epinephrine (n=14) or standard treatment (n=16). According to the study results, there was a significant improvement in coronary blood flow in the epinephrine group: TIMI-3: 28.6% vs 18.8% and TIMI-2: 64.3% vs 12.5% (p=0.004). The difference in the combined endpoint of death+heart failure reached statistical significance: 35.7% vs 81.25% (p=0.01). At the same time, LVEF one day after the procedure in the adrenaline group increased significantly compared to the study before PCI: 44.57 ± 8.20 vs 36.9% ± 13.9% (p=0.01), while the patients of the control group did not demonstrate such positive dynamics: 40.93 ± 34.48 vs 38.31 ± 14.70% (p=0.45) [5].
Another drug with a pronounced coronary vasodilation effect is verapamil. A meta-analysis that included 8 randomized studies with a total of 494 patients showed that intracoronary bolus administration of verapamil/diltiazem provided a statistically significant reduction in the manifestations of the no-reflow phenomenon and a decrease in MACE events within 6 months of observation [6]. The trial aims to estimate the efficacy and safety of the administration of Intracoronary (IC) adrenalin, verapamil, as well as their combination vs standard treatment in patients with STEMI and refractory coronary no-reflow despite conventional treatments during PPCI [7].
Methods/Design
This study is an open-label, randomized, single-center, prospective trial implemented at the cardiology research institute, Tomsk NRMC, Russia. Study intends to assess the safety and efficacy of IC administration of epinephrine and/or verapamil vs standard therapy following onset of severe refractory no-reflow despite conventional pharmacologic and device-based treatment recommended in clinical practice [8]. Consecutive patients with STEMI and refractory no-reflow will be randomized into 4 groups: Standard therapy only, epinephrine, verapamil, epinephrine+verapamil. Intracoronary adrenaline will be administered at a dose of 80 mcg-100 mcg and verapamil at a dose of 0.5 mg [9].
All patients will undergo an assessment of epicardial blood low by TIMI and MBG before and after a bolus of epinephrine and/or verapamil, peak troponin level, ST segment dynamics, echocardiography on 1-3 and 7-10 day, MRI on 2 day, dynamic Single-Photon Emission Computed Tomography (SPECT) on 7 day [10]. A flow diagram describing the temporal phases of standard therapeutic procedures relative to the diagnosis of noreflow onset prior. Patient clinical, laboratory and instrumental data, as well as 30-day clinical outcomes, will be taken from the medical record. Primary endpoints of the study will be mortality rates and new-onset or worsening acute heart failure within 30 days. Written informed consent will be obtained from all patients before the start of the trial (Figure 1) [11,12].
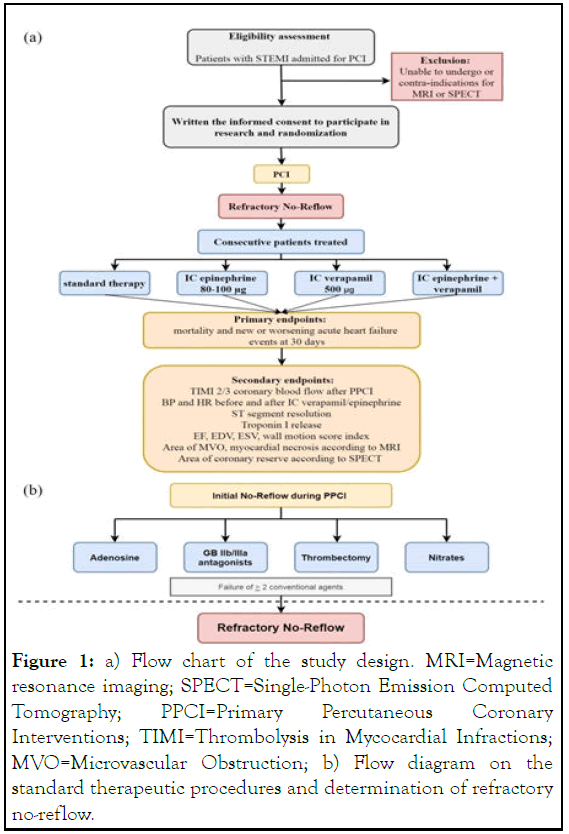
Figure 1: a) Flow chart of the study design. MRI=Magnetic resonance imaging; SPECT=Single-Photon Emission Computed Tomography; PPCI=Primary Percutaneous Coronary Interventions; TIMI=Thrombolysis in Mycocardial Infractions; MVO=Microvascular Obstruction; b) Flow diagram on the standard therapeutic procedures and determination of refractory no-reflow.
Inclusion criteria:
• Patients with ST-elevation myocardial infarction.
• TIMI flow grade 0-1 during the interventional procedure in the culprit vessel after the initial opening of the vessel with the coronary wire.
• Written informed consent to participate in research.
Exclusion criteria (for all groups):
• Inability to undergo or contra-indications for MRI or dynamic SPECT.
Sample size
No sample size calculation was performed as this is a pilot study. A sample size of 40 patients seems sufficient. Baseline and follow-up evaluation is shown in Figure 2.
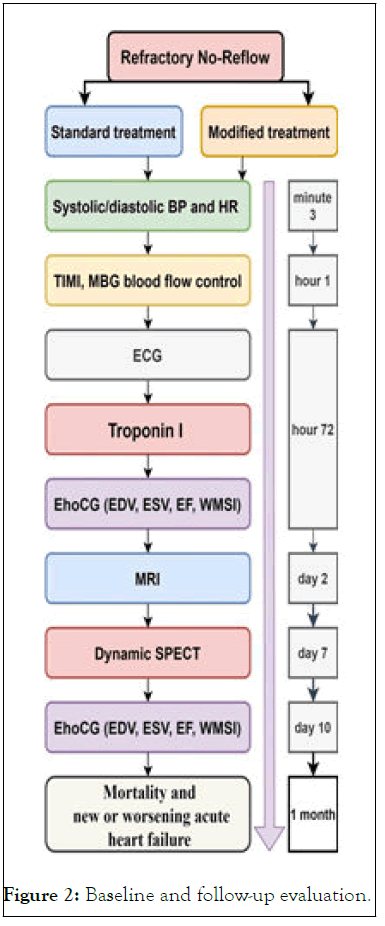
Figure 2: Baseline and follow-up evaluation.
Determinations
Refractory no-reflow will be defined as the coronary severe noreflow episode that did not resolve initially with the combined administration of at least two strategies including nitrates, thrombectomy, glycoprotein IIb/IIIa inhibitors and adenosine.
The MVO assessment will be performed using Magnetic Resonance Imaging (MRI) within two days from PCI. The coronary reserve area will be assessed using dynamic SPECT within the first 7 days. Dynamic SPECT will be performed as follows: The passage of a radiopharmaceutical bolus through the cavities of the heart and myocardium at rest and during infusion of adenosine at a dosage of 140 mcg/kg/min (for 4 minutes) will by recorded. At the peak of the stress test (after 2 minutes of adenosine administration), will be infused 5 ml (dose 260-444 MBq) of 99 mTc-Technetril is administered at a rate of 1 ml/s. Immediately after the end of tracer will by infused 30 ml of 0.9% NaCl. A scintigraphic recording of the study will by begined 5 seconds before the administration of the radiopharmaceutical preparation. On the next day, the study at rest is will be carried out. Scintigraphic images will be recorded in tomographic mode with ECG synchronization for 600 s, at listmode. Image acquisition will be carries out on dedicated cardiac gamma-camera with ultrafats CZT detectors (DiscoveryNM530c, GE Healthcare Israel, Tirat Hacarmel, Israel).
Primary endpoints:
• Mortality events at 30 days.
• New or worsening acute heart failure events at 30 days.
Secondary parameters:
• The rate of patients (percent) who achieved TIMI 2 and TIMI 3 coronary blood flow after primary percutaneous coronary intervention.
• Change in systolic/diastolic blood pressure values and heart rate values before and after intracoronary verapamil/ epinephrine.
• Concentration of troponin I (ng/ml).
• Degree of ST segment resolution on ECG.
• Left ventricular ejection fraction by echocardiography.
• Left ventricular end-diastolic and end-systolic volumes.
• Left ventricular wall motion score index.
• Total volume (mL) of microvascular obstruction, myocardial necrosis, edema, and hemorrhagic impregnation according to MRI data.
• Coronary reserve will be measured by cardiac Single Photon Emission Computed Tomography (SPECT) with technetium 99 m-labeled methoxy-isobutyl isonitrile (99 mТсMIBI) at rest and during pharmacological stress-test (counts).
All data will be statistically analyzed using statistical 10.0 software (Stat Soft Inc., Tulsa OK, USA). Data that are normally distributed will by expressed as mean, standard deviation, minimum value, and maximum value. Data that are nonnormally distributed will by expressed as the lower (q1), median, and upper quartile (q3). Rates and frequencies of occurrence will by expressed as %s (%). Data will by compared between groups using Student's t-test; nonparametric variables will by compared between groups using the Friedman test and median test. A value of P<0.05 is considered statistically significant.
Based on the pharmacodynamic effects of epinephrine and verapamil, their combination is expected to have a more potent vasodilating effect, due to the additive type of synergistic interaction, which will improve coronary microcirculation after PCI in patients with acute myocardial infarction and refractory no-reflow phenomenon. Currently, in clinical practice, there is a possibility of very sensitive diagnosis of Microvascular Obstruction (MVO) using Magnetic Resonance Imaging (MRI), as well as the area of the coronary reserve according to dynamic perfusion scintigraphy of the myocardium. It is advisable to evaluate the effectiveness of treatment of the no-reflow phenomenon using these methods.
Recruitment is ongoing at the time of submission.
The authors certify that they will obtain all appropriate patient consent forms. In the form the patient(s) will give his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
None declared.
VVR, EVV and SVD conceived and designed the study, acquired data, and drafted the manuscript. All other authors acquired data and made substantial contributions and revisions to the manuscript. All authors have read and approved the final manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Dil SV, Ryabov VV, Vyshlov EV (2023) Intracoronary Administration of Epinephrine and Verapamil in the Refractory No-Reflow Phenomenon in Patients with Acute Myocardial Infarction: Study Protocol for an Open-Label Randomized Controlled Trial. J Clin Trials. 13:527.
Received: 04-Dec-2020, Manuscript No. JCTR-20-7402; Editor assigned: 09-Dec-2020, Pre QC No. JCTR-20-7402(PQ); Reviewed: 23-Dec-2020, QC No. JCTR-20-7402; Revised: 02-Mar-2023, Manuscript No. JCTR-20-7402(R); Published: 30-Mar-2023 , DOI: 10.35248/2167-0870.23.13.527
Copyright: © 2023 Dil SV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.