Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2014) Volume 5, Issue 6
A novel method that utilizes untreated and polyurethane foam (PUF) physically immobilized with the reagent 4-(2-pyridylazo) resorcinol (PAR) or 2, 3, 5-triphenyl-2H-tetrazolium chloride (TZ+Cl-) as solid phase extractor packed column has been developed for preconcentration and subsequent determination of titanium(IV) ions in marine and wastewater samples. The method is based on retention of the titanium(IV) traces present in aqueous media at pH 3-4 onto the reagent treated PUF packed column, followed by recovery with HNO3 (2.0 mol dm-3) and subsequent ICP-OES determination. The uptake of titanium species onto the unloaded- and reagent impregnated PUF was fast and followed a first-order rate equation. Titanium sorption onto PUF followed Langmuir, Freundlich and Dubinin- Radushkevich (D-R) type isotherm models. Thus, a dual-mode sorption mechanism involving absorption related to “weak-base anion exchange” and an added component for “surface adsorption” seems a more likely retention model. PAR-immobilized PUF packed column has been applied successfully for complete collection of titanium(IV) species in fresh and wastewater samples at low level of titanium (<0.5 ng Ti mL-1) at pH 3-4. The retained titanium species were then recovered (97-101%) from the packed column with HNO3 and determined by ICP-OES. The proposed PUF packed column method was further applied for analysis of picomolar concentrations of dissolved Ti species in marine water.
Keywords: ICP-OES, Titanium (IV), 4-(2-Pyridylazo) resorcinol(PAR), Polyurethane foam packed column, Preconcentration, Marine and wastewater
Titanium is well known for its excellent corrosion resistance, having ability to withstand attack by dilute H2SO4 and HCl or even moist chlorine. These properties make titanium highly resistant to the usual kinds of metal fatigue. Titanium alloys are principally used for aircrafts and missiles, where light weight strength and ability to withstand extremes of temperature are important. In nature, titanium exists in its most stable and common oxidation state (IV). Many organic compounds of titanium such as phthalates, oxalates, tetraethylate and butyltitanate are widely synthesized and used extensively. Titanium is naturally present in sea and ocean water (picomolar) and in food at only trace levels (μg Kg-1) [1]. The presence of TiO2 as an excipient in most pharmaceutical preparations, pigment, as a particulate food additive and in human intestinal tissue has been proposed that an abnormal response in the pathogenesis of Cohn’s disease additive [2-4]. Hence, determination of titanium (IV) at trace levels in various samples is of paramount importance.
Few methods for the separation and subsequent determination of titanium in food and industrial wastewater samples are known [4]. A reversed-phase liquid chromatographic method for the determination of titanium with 5,5'-methylenedisalicylohydroxamic acid (MEDSHA) has been described by Bagur et al. [5]. Separation and determination of titanium (IV) at trace levels in different matrices including industrial wastewater have been reported employing solid phase extraction (SPE) [6-11]. SPE has several advantages e.g. simple operation, low cost, no time consuming, good selectivity, higher preconcentration factor, rapid phase separation and the ability to be combined with different modern analytical techniques [6-11].
Polyurethane foam (PUF) sorbent in the last four decades UF has been tested as an excellent support in reversed phase extraction chromatography, gas-solid and gas-liquid partition chromatography [12-23]. The cellular structures and the available surface area of the PUF in both foamed and micro spherical forms make it suitable as an excellent extractor and as a column filing material with good capacity for firmly retaining various loading and extracting agents [21]. Preconcentration, separation and subsequent sensitive determination of titanium (IV) in various matrices at trace levels is of prime importance and necessitates. Thus, the present article is focused on: i. studying the retention profile of titanium (VI) onto PAR or TZ+Cl- treated and untreated PUF; ii. Developing a convenient and low cost extraction procedure for separation and subsequent ICP-OES determination of titanium (IV) species in water samples employing PAR immobilized PUF in packed column and finally iii. Assigning the most probable sorption mechanism of Ti retention.
Reagents and materials
All chemicals and solvents used were of analytical reagent grade and were used without further purification. Doubly deionized water was used throughout. Stock solution (0.1 % w/v) of BDH 4-(2-pyridylazo) 4-resorcinol (BDH, Poole, England) and 2, 3, 5-triphenyl-2H tetrazolium chloride ( TZ+Cl-) were prepared by dissolving the required weight in few drops of ethanol and the solution was then completed with water. Stock solution (1 mg mL-1) of titanium (IV) nitrate (BDH) was used for the preparation of diluted solutions (0.05-150 μg Ti mL-1) in water. Stock solutions (1.0 % w/v) of BDH sodium dodecyl sulphate (SDS), tetrabutylammonium bromide, TBA+Br-, (BDH) and Triton X-100 (Analar) were prepared in water. Foam cubes (10-15 mm edge) of commercial white sheets of polyether type based PUF were cut from the foam sheets, purified and finally dried at 80?C [20]. The immobilized reagent PUF cubes were prepared by mixing the dried foam cubes with an aqueous solution (50 mL g-1 dry foam) containing PAR or TZ+Cl- (0.1 % w/v) with efficient stirring for 30 min, squeezed and finally dried as reported [21]. The reagent PAR-immobilized PUF was packed in the glass columns (2 cm, 10 mm ID) by applying vacuum method of foam packing [13,18]. All containers used were pre-cleaned by soaking in HNO3 (20 % w/v) and rinsed with de-ionized water before use.
Apparatus
A Perkin _ Elmer (Lambda 25, Shelton, CT,USA) spectrophotometer (190-1100 nm) with 10 mm (path width) quartz cell was used for recording the electronic spectra and measuring the absorbance of the complex species of titanium. A Perkin Elmer Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES, Optima 4100 DC Shelton, CT, USA) was operated at the optimum instrumental parameters for titanium determination before and after extraction with the reagent treated PUFs under the optimum operational parameters (Table 1). A Soxhlet extractor and a lab-line Orbital mechanical shaker (Corporation Precision Scientific, Chicago, USA) with a shaking rate in the range of 10-250 rpm were used were used for the foam purification and for shaking in batch experiments, respectively. De-ionized water was obtained from Milli-Q Plus system (Millipore, Bedford, MA, USA). A thermo Orion model 720 pH Meter (Thermo Fisher Scientific, MA, USA) was employed for pH measurements with absolute accuracy limits being defined by NIST buffers. Self-made columns (16, 10 and 2 cm h and 10 mm id) were used in flow experiments.
| Rf power, kW | 1050 |
| Plasma gas (Ar) flow rate, L min-1 | 15 |
| Auxiliary gas (Ar) flow rate, L min-1 | 0.2 |
| Nebulizer gas (Ar) flow rate, L min-1 | 0.80 |
| Pump rate, mL min-1 | 1.5 |
| Observation height, mm | 15 |
| Integration time | auto |
| Plasma view | radial |
| Wavelength, nm | Ti 334.940 |
Table 1: ICP-OES Operational parameters of titanium determination by ICP-OES.
Recommended procedures
Batch experiments: In a dry 100 mL polyethylene bottle, an accurate weight (0.05 ± 0.001 g) of the unloaded or PAR immobilized foam cubes was shaken for 1 hr in a mechanical shaker with 50 mL of an aqueous solution containing titanium(IV) ions at 100 μg mL-1 concentration at 25 ± 0.1ºC at the required pH employing Britton-Robinson (B-R) buffer (pH 2.5-11.5). After phase separation, the aliquot solution was analyzed for titanium by ICP-OES under the optimal operational parameters of the instrument (Table 1). The amount of titanium (IV) retained at equilibrium, qe on the foam cubes was then determined from the difference between the concentration of titanium (IV) measured in solution before (Ci) and after (Cf) shaking with the unloaded or reagent loaded foam cubes employing the equation:
qe= (Ci f)v/ w(1)
where v and w are the volume (mL) of the aqueous solution and the weight (g) of the foam cubes, respectively. The extraction percentage (%E) and the distribution ratio (D) of the titanium sorption onto the unloaded and the reagent loaded foam were then calculated as reported [20]. Following these procedures, the influence of different parameters was critically investigated. The values of E and D are the average of three independent measurements and the precision in most cases was ± 2%.
Column experiments: An accurate weight (0.50 ± 0.01 g) of the unloaded or PAR-immobilized PUF was packed in a column using the vacuum method of foam packing [23]. The aqueous solutions (0.1-10 L) containing titanium (IV) at various concentrations (0.01-10 μg mL-1) adjusted with Britton-Robinson buffer of pH = 3-4 were percolated through the foam column at 15-20 mL min-1 flow rate. The sample and blank foam packed columns were then washed with 100 mL of B-R buffer solution at the same pH. Complete retention of titanium (IV) took place on the unloaded and PAR or TZ+Cl--immobilized PUF as indicated from the ICP-OES determination of titanium in the effluent solutions. The sorbed titanium (IV) species were then recovered quantitatively from sorbent packed column with HNO3 (50 mL, 2M) at 5 mL min-1 flow rate. Equal fractions (10 mL) of the eluate were then collected and the titanium species were then determined with ICP-OES.
Analysis of titanium (IV) in fresh, sea and wastewater samples: A 10 mL of concentrated HNO3 was added to 0.1-0.5 L of tap, seawater or industrial wastewater samples. The mixture was boiled until the volume of the sample solution is reduced to two-third and the solution was allowed to cool down and filtered through a Whatman No 1 filterpaper. The pH of the solution was adjusted to 3-4 with B-R buffer. The water samples were then spiked with (or without) titanium (IV) at a total concentration 0.1-10 μg mL-1 and diluted to the original volume with water in a volumetric flask. The water samples were then percolated through the unloaded or PAR-loaded PUF packed column at 20-25 mL min-1 flow rate. The retained titanium (IV) species on the PUF column were then recovered with HNO3 solution (25 mL, 2M) at 5 mL min-1 flow rate. Titanium (IV) concentration before extraction and after recovery in the eluate was finally determined by ICP-OES.
The concentrations of heavy metals in natural water and wastewater samples are frequently lower than their limit of detection (LOD). Therefore, recent years have seen an upsurge of interest in developing solid sorbents [22, 23] and exploring them for the separation and chemical speciation of metal ions [20]. PUF sorbent represents an inexpensive and efficient separation and preconcentration media with steadily versatile applications in inorganic and organic complex species [22].
Retention profile of titanium (IV) onto the PUF
The retention behavior of titanium (IV) ions from the aqueous solutions by the untreated and reagent-immobilized PUF cubes after 1 hr shaking at different pH employing B-R buffer (pH 2.5-11.5) were investigated. The uptake of titanium (IV) onto the unloaded and PAR loaded PUF increases on raising solution upto pH 3.1-4.0 and decreased markedly on increasing the solution pH (Figure 1). The observed behavior of titanium(IV) species sorption onto the unloaded and TZ+Cl-loaded PUF at pH 3.1-4.0 is most likely attributed to the formation of binary and ternary complex ion associates of titanium (IV) with PUFs via protonated ether (-CH2-O+H-CH2-) oxygen linkage of the PUF and TZ+Cl- loaded PUF, respectively. The reagent PAR-immobilized PUF showed also similar trend of sorption with better extraction performance (Figure 1). The low titanium sorption onto PUF at pH higher than pH 4.0 is attributed to the instability, hydrolysis or incomplete extraction of the produced ion associates of titanium (IV) with the unloaded PUF or TZ+Cl-. On the other hand, PAR molecules immobilized onto PUF are most likely complexed with titanium (IV) species in the solution via ligand exchange or ligand addition mechanism [2]. Thus, in the subsequent work, the aqueous solution was adjusted at pH 3-4.
A possible explanation of the observed trend involves a "weak-base anion exchanger" mechanism for the unloaded and TZ+Cl- treated PUF and "cation chelation or ligand addition extraction" mechanism for PAR-immobilized may be preceded [18,20] as follows:
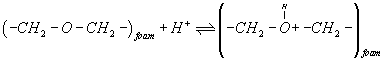 (2)
(2)
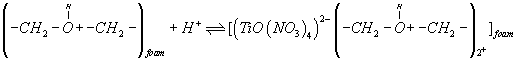 (3)
(3)
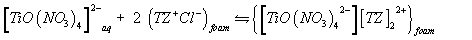 (4)
(4)
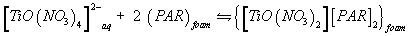 (5)
(5)
The influence of shaking time (1-60 min) on the sorption of titanium (IV) from the aqueous solution at pH 3-4 onto the unloaded and TZ+Cl- or PAR- immobilized PUF was investigated. The extraction was found fast, followed a first-order rate equation and the equilibrium was attained in ~10 min (Figure 2). Thus, a shaking of 20 min time was adopted in the subsequent work. The calculated half-life time (t1/2) of the equilibrium sorption to reach 50% saturation of the sorption capacity of PUF loaded PAR and TZ+Cl- and untreated PUF (Figure 2) was in the range 1.5-2 min. The values of E and D of titanium sorption onto unloaded and PAR-immobilized PUF were found better in comparison with TZ+Cl- immobilized PUF. Thus, in the subsequent experiments, the unloaded and PAR immobilized foams were used.
The effect of cation size (Na+, K+, NH4+ and Ca2+) as chloride salts at concentration 0.05% w/v on titanium sorption by treated and untreated PUF was studied. In the unloaded foam, the uptake followed the sequence:
Na+>K+>NH4+ >Ca2+
Different trend was observed for PAR or TZ+Cl- immobilized, with reasonable increase (5-10 %) of titanium (IV) sorption in the presence of K+ and the retention percentage followed the following order:
K+ >NH4+> Na+≈Ca2+
The reduction of the repulsive forces between adjacent sorbed titanium (IV) complex ion associates in the unloaded PUF membrane may account for the trend observed [22,23]. Thus, the ion-dipole interaction of NH4+with the oxygen sites of the PUF is not the predominating factor in the extraction step. The added K+ ions is most likely reduce the number of water molecules available to solvate the titanium ions which would therefore, be forced out of the solvent phase onto the PUF. Thus, "weak-base anion exchange and "cation chelation or “ligand addition extraction" are the most probable sorption mechanism for the sorbent.
The influence of the surfactants SDS, TBA+Br- and Triton-X 100 on titanium (IV) sorption from aqueous solution onto the unloadedand loaded PUF was investigated. Titanium sorption onto PUF sorbent increased in the presence of SDS (0.1 % w/v) and leveled off on raising the surfactant concentration. This behavior is most likely attributed to the increase of the solution viscosity leading to progressive change in the physical properties of the micro environment of the produced complex ion associates of unloaded or TZ+Cl- treated PUF and the chelate formed with PAR-treated PUF [18,20], respectively. The increase in the solution viscosity enhances the dissociation and/or the formation of aggregate complexes with low diffusion constants [24,25]. The competition between the surfactant and the anionic complex of titanium (IV) may also predominate in the observed trend. Also, the surfactant may reacts directly with the anionic complex of titanium and this may retard the extraction process [25].
Sorption isotherms of titanium (IV) by PUF
The sorption profile of titanium (IV) from the bulk aqueous solution onto the untreated and PAR treated PUF was determined over a wide range of concentrations. In the aqueous, the amount of titanium (IV) retained onto unloaded and reagent treated PUF varied linearly with the corresponding amount of titanium (IV) at low or moderate Ti concentration. Thus, the titanium (IV) sorption onto the PUF were subjected to Freundlich [26] and Dubinin-Radushkevich [27] isotherms over a wide range of equilibrium concentrations. The Freundlich model [26] is expressed as follows:
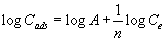 (6)
(6)
where Ce is the equilibrium concentration (M) of titanium(IV) in solution, Cads is the sorbed titanium(IV) ions concentration (mmol g-1) and A and 1/n are the Freundlich parameters related to the maximum sorption capacity of solute (mol g-1). The values of A and 1/n, computed from the intercepts and slopes of linear plots of log Cads versus log Ce over the entire range of titanium(IV) concentrations (0.05-150 μg mL- 1) were 0.0156 ± 0.004, 0.0173 ± 0.003 mol.g-1 and 0.571 ± 0.07, 0.642 ± 0.18 onto the PUF, respectively. The value of 1/n < 1 indicated that, the isotherms do not predict any saturation of the surface of the solid sorbent by the adsorbate and the sorption capacity is slightly reduced at lower concentration.
The linear form of Dubinin-Radushkevich (D-R) model [27] postulated within the adsorption space close to the PAR-Treated PUF adsorbent surface is expressed as follows:
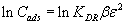 (6)
(6)
where KDR is the maximum amount of titanium (IV) retained onto PAR treated PUF, β is a constant related to the energy of the transfer of the solute from the bulk solution to the solid sorbent and ε is Polanyi potential which is given by the equation:
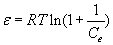 (7)
(7)
where R is the gas constant (kJ mol-1 K-1) and T is the absolute temperature (298 K) in Kelvin. The plot of ln Cads vs. ε2 was linear (Figure 3) indicating that the D-R isotherm is obeyed for titanium (IV) sorption onto the sorbent over the entire concentration range. The computed values of β and KDR from the slope and intercept of Figure 3 were found in the range 0.0027-0.0032 mmol2 kJ-2 and 105-127 μmol g-1, respectively. These results and the data reported earlier [18-20] suggested a dual sorption mechanism involving absorption related to “weak-base anion exchange” or “cation chelation” and an added component for “surface adsorption” mechanism for the uptake of titanium (IV) ions by unloaded and PAR immobilized PUF. This model can be expressed as follows [20]:
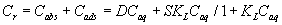 (8)
(8)
where Cr and Caq are the equilibrium concentrations of titanium (IV) ions onto the PUF and in solution, respectively. Cabs and Cads are the equilibrium titanium (IV) ions onto the PUF as an absorbed and adsorbed species, respectively, S and KL are the saturation value for the Langmuir adsorption, the distribution coefficient and the Langmuir constant. This equation can be solved for D as reported earlier [20,21] as follows:
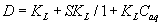 (9)
(9)
The D values are dependent on the titanium ions concentration confirming the proposed mechanism. These results suggested the use of PAR loaded PUF in flow mode for complete collection, recovery and subsequent ICP-OES Ti determination in water.
Chromatographic separation of titanium (IV)
Preliminary investigation on the use of PAR -PUF packed column for the collection of titanium (IV) ions from aqueous media has indicated that, the column performance towards titanium ions is good. Thus, aqueous solutions of deionized and tap water samples (1.0 L) containing various concentrations (0.01-10 μg mL-1) of titanium(IV) at pH 3-4 were percolated through unloaded- and PAR treated PUF packed columns at 20-25 mL min-1 flow rate. Analysis of titanium in the effluent solution versus a reagent blank indicated complete (98 ± 3.1%) retention of titanium. Hence, a series of various eluating agents e.g. HNO3, EDTA, NAF and HCl has been tested for recovery of titanium (IV) from the PUF packed column. Nitric acid (50 mL, 2.0M) was found suitable for complete recovery of titanium (IV) from the packed column at 5.0 ml/min flow rate. The obtainable results are demonstrated in (Figure 4).
The performance of the developed unloaded and PAR-immobilized PUF columns was determined by passing 0.5 L (10 μg mL-1) of titanium (IV) solution at pH 3-4 through the PUF packed column at 20 mL min- 1 flow rate. Complete sorption of titanium (IV) onto PAR loaded foam column took place at 20 mL min-1. The retained titanium (IV) species were recovered with 50 mL HNO3 (2.0M). The results are demonstrated in Figure 5. The height equivalent to theoretical plates (HETP) and the number of plates (N) were then calculated from the elution curves (Figure 5) employing Gluenkauf equation [22]:
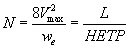 (10)
(10)
where Vmax is the volume of eluting at maximum elution of solute, we is the width of the chromatogram peak at (1/e) times maximum recovery of solute and L is the length of the PUf bed in the packed column. HETP and N values were found in the range 0.5-0.75 ± 0.04 mm and 80 ± 4, respectively. The HETP and N values evaluated from the breakthrough capacity curves (Figure 6) were found also in the range 0.74 ± 0.01 mm; 78 ± 3 (n=5) for titanium retention onto unloaded PUF and 0.51 ± 0.02 mm and 83 ± 2 for PAR-loaded PUF packed column. The critical capacities of titanium (IV) ions sorption onto the unloaded and loaded foam packed column calculated from Figure 6 were 56.3 ± 2.2 and 60.4 ± 1 mg titanium per gram PUF, respectively at 20-25 mL min-1 flow rate. The calculated capacity from Par-PUF packed column was higher than capacity calculated from batch mode (40.4 ± 1 mg/g PUF).
Interference Study
The analytical utility of the PAR-immobilized PUF packed column for the retention and recovery of titanium ions (10 μg mL-1) from aqueous solutions (100 mL) was tested in the presence of a relatively high excess (100-1000 times) of the diverse ions (Fe3+, Al3+, Ca2+, Mg2+,Cr3+, V4+, Ni2+, Mn2+, Co2+, Cu2+, Zn2+, Hg2+, and Cd2+) relevant to waste water. The tolerance less than ± 2% change in the recovery of titanium ions is considered free from interference. Good extraction efficiency (>97 ± 3%) for titanium (IV) ions sorption and recovery were achieved successfully in the presence of the investigated divers ions except Al and Fe. Addition of NaF (100 μg mL-1) prevented the effect of both Al and Fe.
Analytical applications
Analysis of titanium (IV) ions in tap- and wastewater samples: The validity of the proposed unloaded and PAR-loaded PUF packed columns for the collection, recovery and ICP-AES determination of titanium (IV) ions in tap and wastewater samples was tested as described in the experimental procedures. Low concentrations (0.01-1.0 μg mL-1) of the spiked titanium ions in tap and/or wastewater samples were retained quantitatively as indicated from ICP-OES analysis of Ti in the effluent solutions. The retained species of titanium on the unloaded and PAR-PUF columns were then recovered by HNO3 (25 mL, 2M) at 3-5 mL min-1 flow rate and subsequently determined by ICP-OES. The results are summarized in Table 2. Satisfactory results (96.5-102 ± 2.9%) for the recovery of titanium (IV) ions in tap and wastewater samples were achieved by the proposed PUF packed columns and the standard ICPOES. The results revealed absence of Ti in the tested samples in good agreement with the data obtained by ICP-OES method. On these bases, titanium ions are not detectable in tap and the wastewater samples.
| Titanium, µg mL-1 |
Recovery, %* | |||
|---|---|---|---|---|
| Unloaded | PAR-immobilized | |||
| A | B | A | B | |
| 0.05 | 102 ±2 | 100 ±2 | 101 ±2.8 | 101 ±2.6 |
| 0.5 | 99 ±1.5 | 99 ±1.5 | 98.2 ±1.6 | 98.2 ±1.8 |
| 5 | 98 ±1 | 98 ±1 | 98 ±2.4 | 98.5 ±1.7 |
| 10 | 97.5 ±1 | 97 ±1 | 96.5 ±2.9 | 97 ±2.1 |
*Average of five measurements ±relative standard deviation
Table 2: Results of analysis of tap (A) and wastewater (B) samples using the developed unloaded and PAR-immobilized PUF packed columns.
Analysis of titanium (IV) ions in seawater samples: Satisfactory results (97 ± 2.7 %) for the preconcentration, recovery and subsequent ICP-AES determination of very low concentration of titanium (≤ 0.5μgL-1) spiked in Red seawater samples (Jeddah, Saudi Arabia) by the proposed method was attempted. The titanium (IV) concentration (0.05 μgL-1) obtained by the proposed packed column was in acceptable agreement with the data achieved by ICP-mass spectrometry (ICP-MS) and cathodic voltamertric [28] methods.
The present paper demonstrates application of PAR-immobilized PUF solid sorbent packed column for complete removal of titanium (IV) from wastewater samples and subsequent ICP-OES determination. The method is simple to operate and low cost then the conventional method. The PAR-PUF packed column was found stable and it can be reused for many times, without decrease in the extraction and recovery percentage of titanium (over 95%). Work is still continuing for online chemical speciation of inorganic titanium (III) & (IV) and organo-titanium (IV) compounds using PAR-immobilized PUF packed column and ICP-OES.