Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2017) Volume 8, Issue 3
A simple, fast, economical and precise reverse phase liquid chromatographic method was developed, optimized and validated for quantification of naproxen sodium according to the standard guidelines. The separation of the analyte and internal standard was achieved over C-18 column using acetonitrile: water: glacial acetic acid as mobile phase in a ratio of 50:49:1 (v/v) in isocratic mode at a flow rate of 1.2 mL/min at a wavelength of 254 nm at ambient temperature. The retention time was found to be less than 10 minutes. The limit of detection (LOD) was 10 ng/mL and limit of quantification (LOQ) was 15 ng/mL. Naproxen sodium was extracted from biological samples by using acetonitrile as extraction solvent. The linearity was found to be 0.5 to 80 ppm. All the parameters were validated for accuracy, precision, linearity, sensitivity, reproductivity and stability. The method was successfully applied for the quantification of the naproxen sodium in animal and human plasma. Therefore, the method was found to be accurate, reproducible, sensitive, cost effective, less time consuming and can be successfully applied on routine analysis of naproxen sodium in pharmaceutical formulations and pharmacokinetic studies in human and animal models.
Keywords: Naproxen sodium; RP-HPLC; Biological samples; Pharmacokinetics
Naproxen sodium is chemically sodium (2S)-2-(6- methoxynaphthalen-2-YL) propionate. (National Center for Biotechnology Information 2016). Naproxen is a propionic acid derivative that belongs to the aryl acetic group of non-steroidal antiinflammatory agents [1,2]. They act by inhibiting the prostaglandin synthesis thereby producing several side effects such as ulceration and gastrointestinal bleeding [3-5]. Although many high-performance liquid chromatographic methods have been developed for the estimation of naproxen sodium alone [2,5] or in combination for the simultaneous determination of naproxen with other drugs in different matrices. These methods involved derivatization and post-column photochemical reactions, mostly used buffer solution as mobile phase due to which the mobile phase becomes complex. But there is always a need of a meaningful cost-effective method for estimation of naproxen sodium because the present methods do not provide the sensitivity [1,2,5]. The aim of the present study was to develop a simple and effective RP-HPLC method for quantification of naproxen in pharmaceutical preparations [5-9] as well as in spiked human plasma and animal plasma (rabbits) [7,10,11] in a very short time and to validate that method according to ICH and FDA guidelines (Food and drug administration 2012; United states Pharmacopoeia 2007; International Conference on Harmonization (ICH) 2005;). The method was successful due to its suitability and reproducibility. This method is more precise, accurate, highly sensitive, rapid and cost effective when observed under different experimental conditions [12-15]. The dominance of the new assay for its intended use in the human pharmacokinetic study was upheld for its validation and applications over the other methods of naproxen estimation found in literature (Figure 1).
Chemicals and reagents
Naproxen sodium was gifted by Life Pharma, Multan, Pakistan. Acetonitrile and water (HPLC grade) and glacial acetic acid was purchased from Merck (Germany). Other chemicals used were of analytical grade and Naproxen sodium tablet formulations used in this experiment were prepared during research work and a commercial brand was purchased from local market [16].
Instrumentation
High performance liquid chromatographic system equipped with an auto sampler and UV visible detector was used for analysis. The data was recorded using Liquid chromatography software.
Chromatographic conditions
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 15-cm column C-18 that contains 5-μm packing L1. The flow rate is about 1.2 mL per minute. Chromatograph of the standard preparation, and record of the peak responses as directed under procedure: the column efficiency, determined from the analyte peak, is not less than 4000 theoretical plates [17,18].
Mobile phase: A suitable mixture of acetonitrile, water, and glacial acetic acid (50:49:1) was prepared (Make adjustments if necessary. Increased resolution may be achieved by increasing the proportion of water in the mobile phase). The mobile phase was membrane filtered (pore size 0.45 μm), sonicated and degassed before use.
Solvent mixture: A suitable mixture of acetonitrile and water with a ratio (90:10) was prepared (Figure 2).
Preparation of assay
For estimation of naproxen sodium, twenty tablets of 275 mg naproxen sodium tablets were taken randomly, crushed and powdered and the average weight was calculated. The powder equivalent to 2.75 mg of naproxen sodium was taken and transferred to 100 mL volumetric flask and initially dissolved in 10 mL water, and sonicated for 10 minutes until the material was completely dispersed. Then added about 80 mL of acetonitrile, and sonicated for an additional 5 minutes. The flask was allowed to reach room temperature, diluted with acetonitrile to volume, and mix. Any insoluble matter was allowed to settle, then 1.0 mL of the clear supernatant was transferred to a 100 mL volumetric flask; diluted with mobile phase to volume, and mix [19,20].
Preparation of standard solution
An accurately weighed quantity of Naproxen WS was dissolved in solvent mixture to obtain a solution having a known concentration of about 2.5 mg per mL. Then transferred 1.0 mL of the resulting solution to a 100 mL volumetric flask, diluted with mobile phase to volume, and mix. This solution contains about 25 μg of Naproxen WS per mL.
Procedure
Separately injected equal volumes (about 20 μL) of the Standard preparation and the Assay preparation into the chromatograph, recorded the chromatograms, and measured the responses for the major peaks. The relative retention times are around 0.6 for naproxen. The quantity, in mg, of Naproxen (C14H14O3) was calculated in the portion of tablets taken by the formula:
10C (RU/RS)
where, C is the concentration (μg per mL) of Naproxen WS in the Standard preparation, and RU and RS are the ratios of the response of the naproxen peak to the response of peak obtained from the Assay preparation and the Standard preparation, respectively.
The peak area was determined and plotted against concentration μg/mL in plasma. 20 μL was injected into chromatograph and each chromatogram is recorded. The results of calibration curve and system suitability are shown in Table 2 and Figure 3.
Optimization of chromatographic conditions
Various experimental conditions such as different mobile phase, flow rate, detection wavelength and column over temperature were optimized for the analysis of RP-HPLC in isocratic mode. Good separation and shorter run time was obtained by the mobile phase used in varying concentrations of acetonitrile and water to adjust the flow rate to 1.2 mL/min where it gives the improvement in peak shapes of naproxen. Detection wavelength was also varied in the experiment to obtain different chromatograms to achieve better peaks and 254 nm was selected as optimized. Column C-18 well the naproxen by reverse phase chromatography.
Preparation of human plasma samples
Blood samples were collected in heparinized glass tubes from healthy volunteers in University of Lahore, Pakistan after obtaining the informed consent. They were then centrifuged at 3500 rpm for 10 minutes and then filtered through 0.45 μm membrane filter and the plasma is separated and stored at -20°C till analysis. At the time of analysis, the serum was spiked with the naproxen sodium drug solution in a ratio of 1:1 and diluted ten folds to obtain 100 μg/mL. 20 μL of reconstituted samples were injected into the HPLC-UV system [20,21].
Extraction: Naproxen sodium (internal standard) was extracted using acetonitrile ACN. The human plasma was then spiked with the internal standard of naproxen sodium (0.5 μg/mL). The samples were then vortexed for 2 mins and then centrifuged at 3500 rpm for 10 mins. The supernatant was collected and volume make up to 1 mL and 20 μL was injected directly into the system.
Preparation of rabbit plasma samples
Same procedure was adapted for rabbits and samples were collected at a predefined time from the rabbits in heparinized test tubes.
Method validation
The developed method was validated for specificity, accuracy, repeatability, precision, LOD, LOQ, robustness as per ICH guidelines.
Specificity: The difference between API and other excipients present in the sample is called specificity. The specificity was determined by injecting the excipients and standard solution of naproxen sodium.
Accuracy: Accuracy is defined as the nearest of the nominal and the actual results. The accuracy is expressed as percentage recovery of the analyte recovered by the assay. Accuracy was determined by the applying the proposed method to different concentrations of drug product mixtures. The injections were taken in triplicate and mean peak area is taken to calculate the concentration.
The accuracy of the proposed method is calculated by the following equation
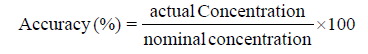
The average accuracy was 98.5% Mean recoveries for naproxen sodium for the specific formulation are shown in table. The results show that are the parameters were within the expected range (Table 1).
| Injected conc. (µg/mL) | Recovered conc. (µg/mL) | % Recovery |
|---|---|---|
| 107 | 105.82511 | 98.9 |
| 120 | 119.1235 | 99.26 |
| 230 | 224.4162 | 97.56 |
Table 1: Accuracy (% recovery) of Naproxen.
Repeatability: The repeatability was done on 3 replicates of each concentration of the standard solution.
Linearity: The linearity plots were obtained over a concentration range of 15 μL to 40 μL to encompass the expected concentration in measured samples. The average peak areas were plotted against respective concentration. The linearity of the proposed method was studied by using the calibration curves (Figure 1) for the determination of the coefficient of correlation and intercept values (Tables 2 and 3).
| Conc. of Standard | Standard ID | Peak Area | Mean | |
|---|---|---|---|---|
| 15 µg/mL | A | A1 | 2942222 | 2941321.67 |
| A2 | 2936694 | |||
| A3 | 2945049 | |||
| 20 µg/mL | B | B1 | 3513865 | 3528324.00 |
| B2 | 3524865 | |||
| B3 | 3546242 | |||
| 25 µg/mL | C | C1 | 4098615 | 4091748.67 |
| C2 | 4087587 | |||
| C3 | 4089044 | |||
| 30 µg/ml | D | D1 | 4630074 | 4650964.00 |
| D2 | 4659300 | |||
| D3 | 4663518 | |||
| 35 µg/mL | E | E1 | 5104615 | 5197315.00 |
| E2 | 5244524 | |||
| E3 | 5242806 | |||
| 40 µg/mL | F | F1 | 5801346 | 5784360.00 |
| F2 | 5753372 | |||
| F3 | 5798362 | |||
Table 2: Linearity Details of Naproxen sodium.
| Standard ID | Concentrations | Peak area (Mean) |
|---|---|---|
| A | 15 µg/mL | 2941321.67 |
| B | 20 µg/mL | 3528324.00 |
| C | 25 µg/mL | 4091748.67 |
| D | 30 µg/mL | 4650964.00 |
| E | 35 µg/mL | 5197315.00 |
| F | 40 µg/mL | 5784360.00 |
Table 3: Linear regression data for naproxen sodium calibration curve.
Limit of Detection and Limit of Quantification: The limit of detection (LOD) was and limit of quantification (LOQ) were determined as per ICH guidelines. The limit of detection (LOD) was 10 ng/mL and limit of quantification (LOQ) was 15 ng/mL (Table 4).
| Spiked conc. | Conc. observed | Accuracy | (%RSD) |
|---|---|---|---|
| 20 | 20.381 | 101.90 | 1.48 |
| 10 | 10.202 | 102.02 | 2.72 |
| 5 | 4.93124 | 98.62 | 2.06 |
| 1 | 1.014 | 101.4 | 1.35 |
| 0.5 | 0.49878 | 99.75 | 0.21 |
Table 4: Determination of naproxen in tablet dosage form by suggested HPLC method.
Precision: Precision was determined through both reproducibility and repeatability. It is defined as the degree of agreement among individual test results where the method is applied repeatedly to multiple readings. Precision is determined by interday and intraday precision. Precision is done by applying the data of three different concentrations on consecutive three days throughout the linearity range thrice in a day. The relative standard deviation for the response factor was calculated. The %RSD value measured during assessment of precision was <2% for naproxen sodium indicating that the method is precise as per ICH guidelines (Tables 5 and 6).
| Spiked conc. | Conc. observed | Accuracy | (%RSD) |
|---|---|---|---|
| 20 | 20.212 | 101.06 | 1.4 |
| 10 | 10.43 | 104.3 | 2.3 |
| 5 | 4.80 | 96 | 1.89 |
| 1 | 1.23 | 123 | 1.24 |
| 0.5 | 0.54 | 108.2 | 2.15 |
Table 5: Determination of naproxen in human plasma by suggested HPLC method.
| Spiked conc. | Conc. observed | Accuracy | (%RSD) |
|---|---|---|---|
| 20 | 20.421 | 102.1 | 2.1 |
| 10 | 9.464 | 94.6 | 0.87 |
| 5 | 5.023 | 100.46 | 1.02 |
| 1 | 0.985 | 98.5 | 1.13 |
| 0.5 | 0.49 | 98 | 0.912 |
Table 6: Determination of naproxen in rabbit plasma by suggested HPLC method.
Sample analysis: To quantify the amount of drug in the finished tablet dosage form, the sample solution of naproxen sodium was analyzed by the proposed method. The contents were determined by the use of calibration curves and the formula is given by,
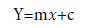
Where, Y=peak area of the analyzed sample; m=slope of the analyzed sample; x=concentration of the analyzed sample and c=intercept of calibration curve.
System suitability: System suitability was conducted in order to confirm the effectiveness of the chromatographic system. Fr this purpose, different suitability parameters such as retention time, tailoring factor, capacity factor (k), selectivity factor (α), resolution, number of theoretical plates were checked by repeatedly injecting the solution of naproxen sodium. The values are given in Table 7.
| Validation parameter | Naproxen |
|---|---|
| Recovery (%) | 98.56 |
| Repeatability (%RSD) | 1.564 |
| Precision range | 0.87-2.87 |
| Interday (n=3) | 1.79 |
| Intraday (n=3) | 1.95 |
| Limit of detection (ng/mL) | 10 |
| Limit of quantification (ng/mL) | 15 |
Table 7: System suitability parameters.
Robustness: The robustness of the method was determined by minor deliberate changes in experimental conditions such as column oven temperature (± 2°C), flow rate of mobile phase (± 0.2 mL/min), composition of mobile phase (± 1). The data obtained after the minor changes in the experimental conditions did not affect the recoveries, peak area and the retention time which showed that the method is robust.
System stability: Stability was tested in an auto sampler for 0, 6, 12 and 24 hr and triplicates of low and high control samples were analyzed and deviation was calculated. Long term stability was tested for two weeks by taking low and high-quality control samples in triplicate. The mean concentration of sample was taken and compared with the samples taken at 0 hr [22] (Figure 4).
This method was a part of an extensive research, “Establishment of in-vitro in-vivo correlation (IVIVC) studies for developed naproxen sodium tablet formulations”. The method was successfully applied to the in-vitro in-vivo evaluation of the pharmaceutical formulations of naproxen in animal models as well as in human plasma. In pharmaceutical development, it is important to employ in-vitro release to select formulations that can provide the adequate therapy. Drug release kinetics provide valuable data on the structural behavior of the drug and its release pattern. The developed method was successfully employed for in-vitro evaluation and pharmacokinetic data of naproxen sodium following IV administration of this drug. The drug content of each sample was determined and the pharmacokinetic parameters were determined by the use of the software.
A reverse phase HPLC method of Naproxen sodium has been developed in tablet dosage form and validated as per ICH guidelines, USP and FDA by using mobile phase of acetonitrile and water in a ratio of 50:49:1 at pH 7.8 using 4.6 mm × 15 cm column that contains 5 μm packing L1 at ambient temperature. The flow rate was 1 ml/ min and the eluent was determined at a wavelength of 254 nm. The retention time of naproxen was 4.699 min (Figure 2). Precision was reflected by mean %RSD of 1.564 which showed the sensitivity of the developed method. A simple extraction procedure was implemented resulting in good recovery of both drug and the internal standard. All the analyte was separated and well resolved from less than 7 minutes. Various experimental conditions such as column over temperature, pH of the mobile phase, detector wavelength, mobile phase ratio and flow rate were optimized for quantitative analysis of naproxen sodium in spiked human plasma and pharmaceuticals and biological samples. The current method is simple, economic, accurate, precise, specific, and rapid and will be conveniently applied for routine analysis in pharmaceutical dosage form, spiked human plasma and biological samples with slight modification in extraction procedures.