Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2017) Volume 8, Issue 3
Monoclonal antibody (mAb) products are extraordinarily heterogeneous due to the presence of a variety of enzymatic and chemical modifications, such as deamidation, isomerization, oxidation, glycosylation, glycation, terminal cyclization, aggregation, and fragmentation. Forced degradation study is a common practice to assess the potential modifications and degradation pathways of mAbs upon extreme conditions, including light exposure and extreme local pH. The degraded samples are subject to characterization using a wide array of analyses, including ion exchange chromatography (IEC) for charge variants and size exclusion chromatography (SEC) for size variants. In this proof-of-concept study, a two-dimensional liquid chromatography (2D-LC) approach was successfully applied to mAb forced degradation samples for evaluation of size distribution in the IEC charge profile. The charge variant peaks in first-dimension (1D) IEC were fractioned by a heart-cutting schedule, and the integrated peak parking feature accommodated later-eluting 1D cuts when the previous ones were still under second-dimension (2D) analysis. Thus, for a singly IEC injection, all IEC fractions were acquired by the 2D for SEC analysis. This study demonstrated that 2D SEC was compatible with 1D IEC for 2D-LC analysis. The method sensitivity was sufficient to determine the aggregates and fragments in each individual IEC cut. The calculated total aggregates and fragments in the stressed mAb-1 samples were comparable to those detected by one dimensional SEC. Most importantly, the 2D IEC-SEC approach was capable of bridging the mAb charge and size variants in a real-time and efficient manner. It was observed that aggregates were enriched in the most basic region in the IEC charge profile, especially in the highly degraded samples. This is the first IEC-SEC 2D-LC application for intact antibody analysis as per the authors’ knowledge.
Keywords: Monoclonal antibody; Two-dimensional liquid chromatography; Ion exchange chromatography; Size exclusion chromatography; Forced degradation; Degradation pathway
Two-dimensional liquid chromatography (2D-LC) is a powerful tool for analyzing highly complex samples. 2D-LC is performed by online transfers of eluent from the first-dimension column to the second-dimension column. Ideally, both columns have orthogonal separation selectivity, thereby increasing the potential peak capacity to the product of the individual peak capacity of each column. 2D-LC has attracted very wide interest in the analysis of small-molecule pharmaceuticals [1,2] and extended its applications to protein characterization in recent years [3,4]. A size exclusion-reversed phase two dimensional-liquid chromatography methodology was developed for stability and small molecule related species in antibody drug conjugates [5]. IEC-RPLC coupled with mass spectrometry detection was applied to identify antibody isoforms directly [6] and to compare originator and biosimilar therapeutic monoclonal antibodies [7]. RPLC-RPLC for HCP analysis [8,9] was reported. Polysorbate 20 analysis in antibody formulation was also explored [10].
Monoclonal antibodies (mAbs) are one of the most successful classes of therapeutics in recent years [11,12]. Forced degradation (FD) studies play a critical role in development therapeutic mAbs [13,14]. More specifically, FD studies provide comprehensive information for candidate selection, process development [15], formulation development [16], assessment of critical quality attributes (CQAs), variant profiling and characterization, comparability studies, stabilityindicating analytical method development and validation, etc. [17-19]. In a FD study, the mAb molecule is intentionally degraded to an appropriate extent by means of various stressing conditions. These conditions include temperature, pH, light, oxidation, chemical agents, mechanical stress, etc. The degraded sample is then subject to characterization using a wide array of biological, biophysical, and biochemical analyses, including different liquid chromatographic methodologies [14]. For instance, ion exchange chromatography (IEC) is commonly used for charge variants analysis [20]. Due to the complexity of the IEC separation mechanism [21], fraction collection and further characterization of IEC variant peaks are usually necessary. Orthogonal methods, such as size exclusion chromatography (SEC), are often applied to the IEC variant characterization. Traditionally, peak fractionation is time and labor consuming. Stability of the fractions may also be a concern. Therefore, it is desirable to analyze IEC variant peaks in a real-time and online manner.
There are two commonly used 2D-LC methodologies, comprehensive and heartcutting. Comprehensive mode typically transfers the entire first dimension (1D) eluent into the second dimension (2D). It is powerful in gaining a comprehensive sample profile within a relatively short time frame. Comprehensive 2D-LC was used for peptide mapping of therapeutic monoclonal antibodies, and three different LC × LC combinations, i.e., strong cation-exchange × reversed-phase (SCX × RP), reversed-phase × reversed-phase (RP × RP), and hydrophilic interaction × reversed-phase (HILIC × RP), were reported [22]. Stolls et al. developed an approach called selective comprehensive multidimensional HPLC that significantly enhanced the resolution of select portions of conventional 1D-LC separations. This approach was then applied for the analysis of opiate compounds and phenytoin and cocaine in wastewater. Heartcutting mode transfers one fraction of interest from the 1D to the 2D. The sampling rate from the 1D is not a concern in heartcutting mode, therefore the 2D method duration can be longer and a gradient method can be used. This effectively increases chromatographic performance, which is critical for low-concentration impurities. Pursch and Buckenmaier applied a loop-based multiple heartcutting 2D-LC methodology to determine the hexabromocyclododecane in polystyrene. The method allowed quantitation of the main isomers which were overlay in 1D-LC. Heartcuts from peaks of the 1D-chromatogram or entire regions of interest are sampled into loops, where they remain parked until their sequential reinjection onto the second dimension column. Matejicek used multi-heartcutting 2D HPLC–MS/MS with a six-port twoposition switching valve equipped with a single loop to determine endocrine disrupting compounds in water. Interestingly, among all the publications, the 2nd dimension always uses reversed phase mainly due to the approach to potential MS application. However, evaluation of aggregation especially in the IEC fractions is not feasible with the IEC-RPLC approach. Here we demonstrate a simple and fast 2D-LC methodology, IEC-SEC to achieve the evaluation of aggregation in the IEC fractions. This is the first IEC-SEC 2D-LC application for intact antibody analysis as per the authors’ knowledge.
Chemicals and materials
MOPS, sodium phosphate dibasic, sodium phosphate monobasic, sodium chloride, hydrochloric acid, and sodium hydroxide were of ACS grade and purchased from Fisher Scientific (Pittsburgh, PA). Water (18.2 MΩ cm) was purified using a Milli-Q filtration system (Millipore, Billerica, MA). IgG1 monoclonal antibody used in this study (mAb-1) was manufactured by Merck & Co. Inc. (Kenilworth, NJ).
2D-LC configuration
The 2D-LC instrument was configured based on two Agilent HPLC systems (Santa Clara, CA). The first dimension (1D) has an 1100 series quaternary pump, an auto-sampler, a thermostatic column compartment (TCC), and a multi-wavelength detector (MWD). The second dimension (2D) has a 1290 series binary pump, a TCC, and a Diode Array Detector (DAD). The two dimensions are interconnected by the multiple heart-cutting (MHC) interface. As shown in Figure 1, this interface includes one 2-position/4-port duo valve in combination with two 6-position/14-port valves. Each 6-position/14-port valve is installed with six 40 μL stainless steel loops. A total of 12 loops therefore can be applied to cut and elute multiple peaks of interest from 1D to 2D. Agilent OpenLAB CDS ChemStation version C.01.04 with 2D-LC add-on software was used for instrument operation and data analysis.
High performance ion exchange chromatography (HP-IEC)
The HP-IEC method used a Dionex ProPac WCX-10 column (4 × 250 mm, 10 μm particle size) from Thermo Scientific (San Jose, CA). The column temperature was maintained at 35°C. A pH gradient program was used to elute the protein. The mobile phase A was 20 mM MOPS at pH 7.2 and the mobile phase B was 60 mM sodium phosphate at pH 8.0. The flow rate was 0.5 mL/min.
Ultra-performance size exclusion chromatography (UP-SEC)
The UP-SEC method used an Acquity BEH200 column (4.6 x 150 mm, 1.7 μm particle size) from Waters (Milford, MA). The mobile phase was 100 mM sodium phosphate buffer at pH 7.0 with 100 mM sodium chloride. The column temperature was maintained at 25°C, and a flow rate of 0.5 ml/min was used.
Antibody stress conditions
For acid stress, mAb-1 was adjusted to pH 3.5 with 0.1N hydrochloric acid. For base stress, mAb-1 was adjusted to pH 10.0 with 0.5N sodium hydroxide. The sample aliquots were incubated at 25°C for 1, 2, 4, and 7 days, respectively. At the end of each time point, the aliquots were buffer exchanged into formulation buffer. Control samples were prepared by buffer exchange right after pH adjustment with no incubation. Light stress of mAb-1 was conducted in a Caron photo-stability chamber (model 6545-2, Marietta, OH). The exposure levels are 0.2X, 0.5X and 1X. According to ICH Q1B, X refers to light exposure to 1.2 million lux hours of white light and 200 watt hours per square meter of UV light. The chamber temperature was maintained at 25°C. The dark control sample (0X) was wrapped with aluminum foil and kept in the chamber during the whole light exposure period. The concentration of mAb-1 was 10 mg/mL in all stress conditions.
1D UP-SEC for size variant analysis
Molecular size based separation methods are integral components of quality control strategies of therapeutic antibodies. MAbs present size heterogeneity including aggregation and fragmentation. SEC is the long-standing standard in the biopharmaceutical industry for detection and quantitation of protein impurities, particularly soluble aggregates. Figure 2 shows a representative UP-SEC chromatogram of mAb-1. The aggregates peak elutes earlier than the main peak while the fragments peak elutes later. Figure 3 presents the change of size profile of mAb-1 under stress conditions. In a time course of seven days, the percentage of aggregate decreased from 1.0 to 0.5 under acid stress, while the percentage of fragments increased from 0.1 to 0.6. Upon base stress, the aggregates percentage increased significantly from 1.1 to 4.2 after seven days, while the fragments amount did not change much. Light stress caused both aggregates and fragments to increase almost linearly. As indicated by the slopes, 1X of light stress could induce about 3.7% increase of aggregation, and about 0.5% increase of fragmentation, respectively.
1D HP-IEC for charge variant analysis
Charge-based separation techniques are also critical components of quality control strategies of therapeutic antibodies. IEC preserves the native conformation and maintains the biological activity of the protein, therefore has been a popular method for charge variant analysis in the biopharmaceutical industry. Figure 4 upper panel presents a representative chromatogram of mAb-1 using the cation exchange (CEX) separation mechanism. The most abundant peak is referred to as the main species. The peaks eluting earlier pose lower isoelectric points (pI) compared with the main species and are generally referred to as acidic variants. The later-eluting peaks have higher pI values compared with the main species and are referred to as basic variants. Charge variant profiling of intact antibody provides diverse information for protein conformation, size, sequence specifics, glycosylation, and post-translation modifications. Understanding the chemical nature of main, acidic and basic species, and the differences among these three species, is critical for process development and formulation design. Charge variant profile is also a stability-indicating attribute. Under stress conditions such as pH, light exposure and/or elevated temperature, the charge profile changes are correlated to the stress levels or stress duration. Figure 5 shows such correlations. For samples stressed at pH 3.5, the percentage of acidic variants remained relatively constant, while the percentage of basic variants increased slightly over time. Correspondingly, the percentage of main peak decreased slightly over the period of seven days. For samples stressed at pH 10.0, the percentage of acidic variants increased dramatically while the amount of basic variants did not change much.
The percentage of main peak decreased significantly after seven days. Light stress caused both acidic and basic variants to increase over the duration of exposure, and the main peak decreased correspondingly. Peptide mapping analyses of the stressed mAb-1 samples (data not shown) revealed that the major degradants include deamidation products upon basic stress, methionine oxidation products upon light exposure, and crosslinking (thioether linkage) products upon both acid and base stresses. These degradation products can be source of charge related heterogeneity of therapeutic mAbs (Khawli 2010) as well as the source of aggregation. In this study, we demonstrate that 2D-LC is capable of bridging the charge and size variants and hence facilitate a deeper understanding of the protein degradation pathways.
Multi heart-cutting (MHC) 2D-LC with the peak parking feature
The first dimension chromatography used IEC. As shown in Figure 4 upper panel, eleven cuts were transferred from 1D to 2D for SEC analysis through the MHC interface. Cuts 1 to 5 were from the acidic region, cuts 7 to 11 were from the basic region, and cut 6 was from the main peak. The transfer time of each cut was 0.08 minute. Since the flow rate was 0.5 ml/min for IEC, the transfer volume of each cut was 40 μL. The 2D SEC cycle time was four minutes. Figure 4 lower panel presents a full picture of the 2D SEC chromatogram.
The peak parking feature of the MHC interface was utilized to accommodate later-eluting 1D cuts when the previous ones were still under 2D analysis. Table 1 summarizes the retention time of each 1D IEC cut as well as the corresponding 2D SEC start time. Most cuts were parked in the sampling loop prior to the 2D SEC analysis. Additionally, due to the built-in valve-switching algorithm of the MHC to prevent from contamination, 1D cuts parked earlier might be run later in the 2D analysis.
| Cut# | 1D Cut start (min) | 2D Run start (min) | SEC peak# | Peak-parking |
|---|---|---|---|---|
| 1 | 10.7 | 10.8 | 1 | No |
| 2 | 15.5 | 15.6 | 2 | No |
| 3 | 16.7 | 27.6 | 5 | Yes |
| 4 | 18.6 | 23.6 | 4 | Yes |
| 5 | 19.5 | 19.6 | 3 | No |
| 6 | 21.0 | 51.6 | 11 | Yes |
| 7 | 22.0 | 47.6 | 10 | Yes |
| 8 | 25.2 | 43.6 | 9 | Yes |
| 9 | 26.0 | 39.6 | 8 | Yes |
| 10 | 27.9 | 35.6 | 7 | Yes |
| 11 | 31.5 | 31.6 | 6 | No |
Table 1: 2D-LC 1D Heart-Cut Start Time and 2D Run Start Time.
Characterization of FD samples using IEC-SEC 2D-LC
MAb-1 FD samples were analyzed using the IEC-SEC 2D-LC method. The relative percentage of aggregates in each 1D IEC cut was determined by 2D SEC. The results are summarized in Figure 6. Interestingly, aggregation was not detected in the cuts from the acidic region, i.e., cuts 1 to 5, and was at trace level in the main species, i.e., cut 6. While in cuts 7 to 11, aggregates were generally detected but enriched in the most basic region. Cut 11 had the highest relative percentage of aggregates in the initial samples. The level ranged from 15.1% to 17.4%. Cut 10 had the second highest relative percentage of aggregates. The level ranged from 3.3% to 6.6%. When mAb-1 was stressed with acid for seven days, the aggregation level decreased gradually from 6.3% to 2.4% in cut 10, and decreased from 15.1% to 7.1% in cut 11. While in the base and light stressed samples, respectively, the aggregation levels increased dramatically over time. Seven days of basic stress led to an aggregation increase from 6.6% to 37.9% in cut 10, and from 17.3% to 38.0% in cut 11.
In the sample exposed to 1X light, an increase of aggregates was observed from 3.3% to 24.1% in cut 10, and from 17.4% to 41.3% in cut 11. These trends are in good agreement with the 1D SEC results but the magnitude of change is much greater. As the aggregation levels were not high in cuts 7 to 9, no obvious trend was observed. The enrichment of aggregates in the most basic region of mAb-1 was explained by the reduced surface charge of aggregate molecules. However, further studies may be necessary to fully understand the retention mechanism of protein aggregates on IEC columns. Compared with aggregation, fragments were generally detected in the main and acidic region, as indicated in Figure 7. While, there was no obvious trend observed probably due to the relative low abundance of fragmentation in mAb-1 and its FD samples.
Assuming the aggregation percentage in each cut determined by 2D SEC represents the average level of aggregates in the corresponding 1D IEC peak, the total amount of aggregates across the whole IEC profile, referred to as %Aggregates2D, can be calculated using the following equation:
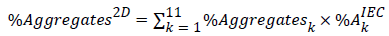 Eq. 1Here %Aggregates K refers to the aggregation percentage in cut k measured by 2D SEC, and
Eq. 1Here %Aggregates K refers to the aggregation percentage in cut k measured by 2D SEC, and 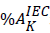 is the corresponding IEC peak percentage relative to the whole IEC profile. Similarly, fragmentation can also be calculated using the following equation:
is the corresponding IEC peak percentage relative to the whole IEC profile. Similarly, fragmentation can also be calculated using the following equation: 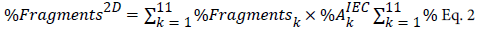
Where %Fragments2D refers to the total amount of fragments across the whole IEC profile, and %FragmentsK is the fragmentation percentage in cut k. Comparing %Aggregates2D and %Fragments2D with the size variant results obtained from Σ11om 1D SEC, the accuracy of the 2D IEC-SEC method can be assessed. As shown in Table 2, the results are quite comparable, indicating that the 2D sensitivity is sufficient to determine the accurate level of aggregates and fragments in each IEC cut.
| Stress | Percentage | Technique | Day 0 | Day 1 | Day 2 | Day 4 | Day 7 |
|---|---|---|---|---|---|---|---|
| Acid | Aggregates | 1D SEC | 1.0 | 0.7 | 0.7 | 0.7 | 0.5 |
| 2D IEC-SEC | 0.9 | 0.6 | 0.7 | 0.6 | 0.5 | ||
| Fragments | 1D SEC | 0.1 | 0.2 | 0.4 | 0.4 | 0.6 | |
| 2D IEC-SEC | ND | 0.2 | 0.3 | 0.3 | 0.6 | ||
| Base | Aggregates | 1D SEC | 1.1 | 1.9 | 2.4 | 3.1 | 4.2 |
| 2D IEC-SEC | 1.0 | 1.6 | 2.1 | 2.9 | 3.8 | ||
| Fragments | 1D SEC | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | |
| 2D IEC-SEC | ND | ND | ND | 0.1 | 0.2 | ||
| 0 X | 0.2 X | 0.5 X | 1 X | ||||
| Light | Aggregates | 1D SEC | 0.8 | 1.6 | 2.6 | 4.5 | |
| 2D IEC-SEC | 0.8 | 1.5 | 2.3 | 4.4 | |||
| Fragments | 1D SEC | 0.1 | 0.2 | 0.3 | 0.6 | ||
| 2D IEC-SEC | 0.1 | 0.2 | 0.3 | 0.6 |
Table 2: Comparison of aggregation and fragmentation results by 1D SEC and 2D IEC-SEC.
In this proof-of-concept study, two-dimensional IEC-SEC was successfully applied to mAb forced degradation samples for evaluation of size distribution in the IEC charge profile. The charge variant peaks in 1D IEC were fractioned by the heart-cutting schedule, and the integrated peak parking feature accommodated later-eluting 1D cuts when the previous ones were still under 2D analysis. Thus, for a singly IEC injection, all IEC fractions were acquired by the 2D for SEC analysis. This study demonstrated that 2D SEC was compatible with 1D IEC for 2D-LC analysis. The method sensitivity was sufficient to determine the aggregates and fragments in each individual IEC cut. The calculated total aggregates and fragments in the stressed mAb-1 samples were comparable to those detected by one dimensional SEC. Most importantly, the 2D IEC-SEC approach was capable of bridging the mAb charge and size variants in a real-time and efficient manner. It was observed that aggregates were enriched in the most basic region in the IEC charge profile, especially in the highly degraded samples. Twodimensional liquid chromatography is a valuable tool to bridge orthogonal methods for protein characterization, and we are continuing our evaluation for other LC method combinations, for instance, distribution of oxidants in charge variants by 2D IEC-HIC.
The authors thank Bob Giuffre, Joon Lee, and Monica Pirone from Agilent for installation and technical support of the 2D-LC instrumentation.