Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2023)Volume 11, Issue 1
The aim of this study was to evaluate the impact of fermentation by single cultures of Lactobacillus casei, Lactobacillus rhamnosus, Bifidobacterium bifidum and Saccharomyces cerevisiae and their multiple co-cultures on the different physiochemical and nutritional parameters of wheat. Fermentation by Lactobacillus casei proved to increase the protein content (27% w/w) as compared to (control) unfermented wheat (12.7% w/w). All probiotics cultures drastically reduced the phytic acid content particularly Lactobacillus casei reduce the level from 1269 mg/100 g to 127 mg/100 g. After fermentation both essential and non-essential amino acids were increased, single culture of Saccharomyces cerevisiae showed higher amount of valine and methionine (187.24 mg/g) and (135.71 mg/g) respectively. Lipid content was increased by Lactobacillus casei (23%) compare to control (2.5%). Antioxidant activity was also increased after the fermentation by Lactobacillus casei with the combination of Saccharomyces cerevisiae (78.60 ± 2.12%).Hence the results showed that fermentation of wheat grains by different probiotics is adequate to increase the nutrient value and reduces the anti-nutritional factors and enhance the utilization of wheat in food systems.
Wheat; Fermentation; Amino acids; Protein; Probiotics; Phytic acid
Among cereals, wheat (Triticum Aestivum) is the second most important crop which is consumed world [1]. Among all the nutrients present in wheat, protein plays an important role in maintaining the quality of wheat [2]. Most of the wheat species contain 9%-13% of protein content but can be improved up to 25%-30%. The stability of amino acid in wheat grain is very unsound; mainly total essential amino acid content [3]. Generally lysine, threonine, and isoleucine are among limiting amino acids in wheat, which can not suffice the dietary requirements of population [4]. Thus, it has become compulsory to increase the percentage of different nutrients especially proteins in wheat grains by improving the amino acid composition. There are many different methods to enhance the quality of protein and amino acid composition of wheat protein but fermentation is one of the most cost-effective and prehistoric methods to increase the nutritive value of as well organoleptic property of any food product [5]. Certainly, fermentation decreases carbohydrates and some oligosaccharides and polysaccharides but at the same time, it increases different essential amino acids like methionine and lysine as well as vitamins particularly of group B, mainly (Riboflavin) Vit-B2, (Niacin) Vit-B3 [6-8].
Anti-nutritional components that are known as food inhibitor such as phytic acid that prevents the bioavailability of minerals such as calcium, magnesium and iron for monogastric animals including humans as they lack phytase enzyme in their digestive tract [9]. Phytates in the form of phytic acid are present in the wheat grains that produce insoluble salts with minerals such as calcium, magnesium and iron. Production of such salts reduces the bioavailability and absorption of these minerals in the human body [10]. Fermentation can attain reduction in phytic acid in cereals to a large extent by the action of microbial cultures and grain phytases. Phytases developed during the fermentation by probiotics decreases the hexa form of phytic acid into lower forms, such as IP1, IP2, IP3, IP4, IP5 and myo-inositol [11]. Thus, lower forms of phytic acid have a lower binding capacity for metals like iron, calcium and zinc [12]. Near about 88.3% decline in phytate content was documented when germinated pearl millet sprouts were fermented with mix pure cultures of Saccharomyces diasticus, S. cerevisiae, and Lactobacillus strains [13]. Thus fermentation and other different process such as germination, soaking or treatment of cereals with enzymes such as phytase enzyme can be used to decrease these anti-nutritional components. Food fermentation of enhancing the nutritive quality of cereals, these foods are known to be the important part of the diet. Lactic acid bacteria are most important bacteria used for fermentation of cereals and are known to maintain the intestinal health, bone health, blood cholesterol levels etc., [14]. Fermentation by single culture or mixed co-culture can be therefore utilized as useful tool for improving the nutritional quality and amino acid profile of wheat. Thus, the present study deals with enhancement of nutritional value of wheat by fermentation using different probiotics and use of fermentation to reduce the anti-nutritional factors and improving their absorption and bioavailability to humans. The effect of fermentation by different probiotics on pH and antioxidant activity is also investigated.
Methods for purification of novel bacteriocins
Microbes used for the fermentation were procured from Unique Biotech, Hyderabad. Three microbes were used viz. Lactobacillus casei, Bifidobacterium bifidum, Lactobacillus rhamnosus with their strain no. UBLC-42, UBBB-55, UBLR-58 respectively and yeast Saccharomyces cerevisiae was obtained from Sigma Aldrich with CAS No-9012-72-0. Wheat variety Shalimar wheat was procured from SKUAST-K (Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir) under registration no-Au/MRCFC/FS/332.1,1-Diphenyl-2-picrylhydrazyl (DPPH), (CAS No:1898-66-4), Ascorbic acid (CAS No:50-B1-7) and phytic acid sodium salt hydrate (CAS No:83-86-3) were purchased from Sigma Aldrich, India. The 20 standard amino acids (Tyrosine, Alanine, Arginine, Asparagine, Aspartic acid, Cysteine, Glutamine, Glutamic acid, Glycine, Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Valine) were procured from Sisco Research Laboratories (SRL), New Delhi. All the chemicals and reagents were of analytical grade.
Sample preparation
The wheat grains (500 g) were washed and soaked in deionized water (1 L) for 12 hours at 25°C. After soaking wheat grains were cleaned with new water and softened wheat was ground in grinder (HL7505, Philips, India) and bran was detached by filtering through the muslin cloth. The collected residue was transferred into the 50 ml of different Erlenmeyer flasks and autoclaved for 15 mins at 121°C at 15 psi followed by cooling for 15 mins. Different probiotics such as Lactobacillus rhamnosus, Bifidobacterium bifidum, Lactobacillus casei, and yeast Saccharomyces cerevisiae and their mix co-culture were utilized for the fermentation. 10 ml of the extract was prepared by scraping the surface of petri-plate with loop from all different probiotic cultures and then 1 ml of the extract from each were used for inoculation of grinded wheat and were fermented for 48 hours at 37°C (Orbital incubator, Metrex scientific instruments (P) LTD, New Delhi). Drying was carried in tray drier below 40°C for 8 hours and dried powder is referred to the final product.
Proximate composition and physicochemical parameters of fermented and unfermented wheat
Previously described method by Association of Official Agricultural Chemists (AOAC) was used for the determination of protein, moisture, fat, fiber, ash content. While as carbohydrate content was determined by difference. The pH of the both fermented as well as unfermented wheat was measured in duplicates by using a digital portable pH-meter equipped with a penetration probe (Auto Digital pH meter, Khera instruments Pvt. Ltd).
Antioxidant activity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH) assay
Free radical scavenging activities of all the samples were determined by using 1,1-Diphenyl-2-picrylhydrazyl (DPPH) according to Chen, et al [15]. The sample solution (2 mg/ml; 1 mL) were prepared by adding it to 4 mL of a 0.004% methanolic solution of DPPH. The prepared samples were incubated at room temperature in the dark place for 30 min. The optical absorbance of samples was read at 517 nm using a spectrophotometer. Ascorbic acid was used as a standard control. To calculate DPPH free radical scavenging activity, the percentage inhibition graph was plotted between the different concentrations of probiotic strains from 15.62 µg/mL-500 µg/mL.
Determination of phytic acid
Phytic acid reduction was determined by spectrophotometric method in which sample and standard solutions were prepared in 0.75 mol L-1 of Nacl 20 mL of HCl (0.5 mol L-1) was added to 0.5 g of each sample and was maintained at constant agitation for 1 hour [16]. Samples were centrifuged at 4000 rpm for 30 min and supernatant was filtered and collected. Absorbance of sample and standard samples were measured at 500 nm after min of sample preparation.
Amino acid analysis
Preparation of the amino acid sample for High Performance Thin Layer Chromatography (HPTLC) analysis: The standard solution of the amino acid was prepared in solutions 70% ethanol. Final concentration was adjusted to 1 mg/ml using High Performance Liquid Chromatography (HPLC) grade ethanol. The wheat samples were weighed accurately and homogenized in water. The mixture was centrifuged at 3000 rpm for 10 minutes. The supernatant was taken and dissolved in 70% ethanol (1:4). The volume of the total mixture was measured and centrifuged once more at 3000 rpm for 10 minutes. The supernatant was discarded and pellet formed was taken. The pellet obtained was hydrolyzed in 6 N HCl at 100°C for 2 hours. The red color was produced and 6 N NaOH was added for neutralization. The mixture was filtered through Whatman filter paper No.1 and the filtrate was used for HPTLC analysis. The standards samples were applied to HPLTC machine (CAMAG, Linomat-V Switzerland), 5 μl of each filtered extract were applied individually as 6 mm wide band to pre-washed and activated silica gel 60 F254 pre-coated HPTLC plates (20 × 10 cm; Merck, Germany) with the nitrogen flow providing a delivery speed of 150 nL/s from the syringe. The TLC plates were developed with a solvent system consisting of n-butanol/acetic acid/water (3:1:1) Reference. The developed plates were stained using 0.3% ninhydrin in n-butanol as spraying reagent and the plates were heated at 110°C for 10 min. These plates were scanned digitize analyzed by using CAMAG software. The Ultraviolet (UV) detection was performed densitometrically at maximum absorbance wavelength, 490 nm by Thin Layer Chromatography (TLC) scanner III using Wincats1.2.3 software at 610 nm [17].
Statistical analysis
Statistical representation of the data were expressed as mean ± SD using One Way ANOVA followed by Tukey test to compare all the pairs of the column. The statistical significance difference was represented in terms of p value and summary. Control (unfermented) was compared with the fermented samples. P-values <0.001, <0.01, <0.05 were reflected as statistically different.
Proximate chemical analysis
The proximate composition of unfermented wheat (control) and wheat fermented by different probiotics is reported in Figure 1. As it can be seen in the figure, the moisture content is decreased in the wheat fermented by Lactobacillus casei as compared to the control (unfermented wheat) sample, while as Lactobacillus rhamnosus also revealed the decrease in moisture content. The reduction analysed in moisture content is due to increase in dry material content because of proliferation in microbial cell. Obadina, et al. [18] also reported the decrease in moisture content in soymilk fermentation. Results were also in line with Lund [19] that observed the elevation in the concentration of nutrients due to the decrease in moisture content (Figure 1).
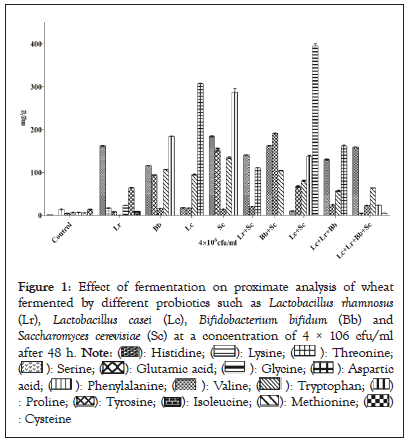
Figure 1: Effect of fermentation on proximate analysis of wheat fermented by different probiotics such as Lactobacillus rhamnosus (Lr), Lactobacillus casei (Lc), Bifidobacterium bifidum (Bb) and Saccharomyces cerevisiae (Sc) at a concentration of 4 × 106 cfu/ml after 48 h.
On the other hand the lipid content varied from 23% to 2.5% in wheat fermented by Lactobacillus casei compared to the control sample respectively. It was also increased in wheat fermented by single culture of Saccharomyces cerevisiae i.e. 16.4%. Increase in lipid content may be due to breakdown of complex fat molecule to simpler ones extensively, it is due to activity of lipolytic enzymes that can result in increase of lipid content [20]. However it may vary according to the probiotics used in fermentation. The results were similar with those of Abu, et al. [21] that reported increase in lipid content of sweet potato fermented by Aspergillus oryzae.
The protein content was found to increase from 12.7% to 27.0%. Lactobacillus casei with the combination of Saccharomyces cerevisiae showed highest increase in the protein content while as protein content was found lower in Lactobacillus casei with combination of Lactobacillus rhamnosus and Bifidobacterium bifidum followed by Bifidobacterium bifidum with the combination of Saccharomyces cerevisiae 5.18% and 5.56% respectively. Increase in protein content was also reported in Cocoyam [22]. The increase in microbial mass during fermentation that leads to hydrolysis of protein molecules into amino acids and into few peptides may be attributed to the increase of protein content due to fermentation [23]. According to Shang, et al. [24] it is observed that protein offers the basic structure of bone and help to bind the other minerals. Protein is responsible for the formation on one-third of bone mass and undergoes further resorption and bone formation [25].Thus protein acts as a key nutrient for maintaining the bone health. Thus, Lactobacillus casei and Saccharomyces cerevisiae are responsible in increasing the protein content in the wheat.
Lactobacillus casei is also responsible in decreasing the fiber content by 1.75% in fermented wheat followed Lactobacillus rhamnosus with the combination of Bifidobacterium bifidum and Lactobacillus casei by 2%. These findings were similar with Igbabul, et al. [22] in which fiber content was also decreased. Fiber reduction is an indication of softening the fibrous tissues in fermented products during the process of fermentation and low fiber content in this study may be due to the bioconversion of carbohydrates and lingo-cellulose into protein by activities of probiotics and were in line with results of Bau, et al [26]. But the combination of Lactobacillus rhamnosus with the Saccharomyces cerevisiae resulted in the increase in the fiber content by 10.1%.
Carbohydrate content of unfermented wheat was recorded in the range of 62.3% while as comparing to wheat fermented by Lactobacillus rhamnosus with combination of Saccharomyces cerevisiae it was found to be 31.5% followed by 32.3% in wheat fermented by Bifidobacterium bifidum, and 35.2% in wheat fermented by Lactobacillus casei as shown in Figure 1. Fermentation significantly decreases the carbohydrate in the wheat fermented by probiotics and their combinations, this reduction may be due to the utilization of fermentable sugars by lactic acid bacteria for the growth, survival and other activities of probiotics [27].
pH analysis
The changes in pH in fermented and unfermented wheat are represented in Figure 2. pH of unfermented wheat (control) was observed 6.77 and 4.1 in Lactobacillus casei with the combination of other three probiotics. The pH values were decreased to 3.20 in wheat fermented by Saccharomyces cerevisiae and 3.33 in its combination with Lactobacillus rhamnosus. pH was also decreased by other probiotics such 3.50 by Lactobacillus casei, 3.63 by Lactobacillus rhamnosus, and 3.86 in wheat fermented by Bifidobacterium bifidum. The decrease in pH in fermented samples of wheat is attributed to the activities of probiotics particularly lactic acid bacteria. These results are in line with A. O. Ojokoh, et al [28] (Figure 2).
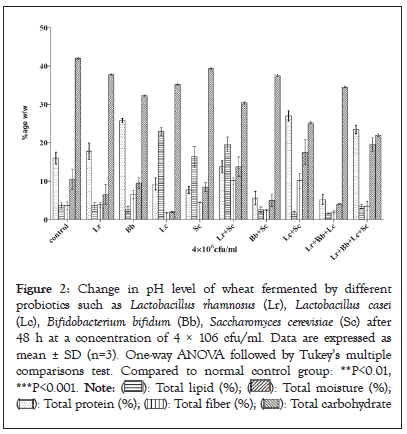
Figure 2: Change in pH level of wheat fermented by different probiotics such as Lactobacillus rhamnosus (Lr), Lactobacillus casei (Lc), Bifidobacterium bifidum (Bb), Saccharomyces cerevisiae (Sc) after 48 h at a concentration of 4 × 106 cfu/ml. Data are expressed as mean ± SD (n=3). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: **P<0.01, ***P<0.001.
Antioxidant activity
The antioxidant activity of both fermented wheat and unfermented wheat (control) was determined by DPPH scavenging activity as shown in Figure 3. The highest antioxidant activity was measured as 78.60 ± 2.12% in wheat fermented by Lactobacillus casei with the combination of Saccharomyces cerevisiae while as the combination of three probiotics Lactobacillus casei, Bifidobacterium bifidum and Lactobacillus rhamnosus exhibited the lowest value antioxidant activity 34.10 ± 1.41% as compared to standard ascorbic acid 89.17 ± 1.23% at the concentration of 500 µg/ml. Antioxidant activity of wheat was improved after fermentation with Lactobacillus species. The improvement in the antioxidant activity of wheat might be due to the increase in the protein content as reported by Wu, et al [29] (Figure 3).
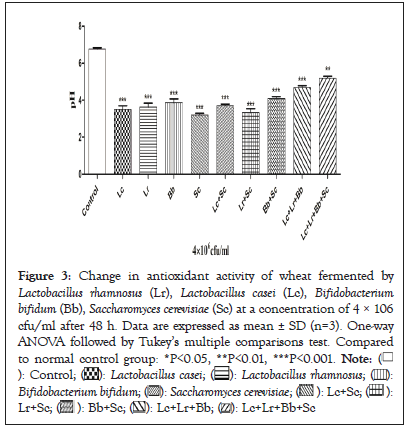
Figure 3: Change in antioxidant activity of wheat fermented by Lactobacillus rhamnosus (Lr), Lactobacillus casei (Lc), Bifidobacterium bifidum (Bb), Saccharomyces cerevisiae (Sc) at a concentration of 4 × 106 cfu/ml after 48 h. Data are expressed as mean ± SD (n=3). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: *P<0.05, **P<0.01, ***P<0.001.
Phytic acid analysis
The effect of fermentation on the anti-nutritional factor of the fermented as well as unfermented wheat is represented in Figure 4. It was observed that phytic acid is decreased after fermentation and there was significant difference between unfermented wheat and fermented wheat samples. In this study, phytic acid was reduced by all probiotics, but phytic acid content of the wheat fermented by Lactobacillus casei reduces from 1269 mg/100 g to 127 mg/100 g. Further decrease in phytic acids is due to fermentation by different probiotic culture. Single culture of Lactobacillus rhamnosus reduced the level of phytic acid to 241.8 mg/100 g while as its combination with another culture Saccharomyces cerevisiae eliminates the phytic acid content to lower levels (118.2 mg/100 g). Combination of multiple cultures may help in eliminating the phytic acid completely. Bifidobacterium bifidum and its combination culture with Saccharomyces cerevisiae also decreases the activity of phytic acid to 215.8 mg/100 g to 212.1 mg/100 g while as single culture of Saccharomyces cerevisiae reduces the level to 137.9 mg/100 g. The activity of endogenous phytase enzyme from the unfermented wheat and essential added probiotics are capable of converting the phytic acid into inositol and orthophosphate during the fermentation. Bioavailability of minerals is enhanced in fermented cereals when fermented by yeast because of hydrolysis of phytic acid. Factors such as pH and temperature are also interrelating in the reduction of phytic acid. In this study, fermentation was carried at 37°C to reduce the phytic acid content, as the optimum temperature regarded for phytase activity is between the ranges of 35°C to 45°C. Decrease in the anti-nutritional components during the process of fermentation has been also studied earlier in different fermented foods [30]. Some studies also reported that carbon dioxide and organic acids increase the solubility of phytic acid and phytase activity because of low pH during the fermentation thus reduces the phytic acid content [31] (Figure 4).
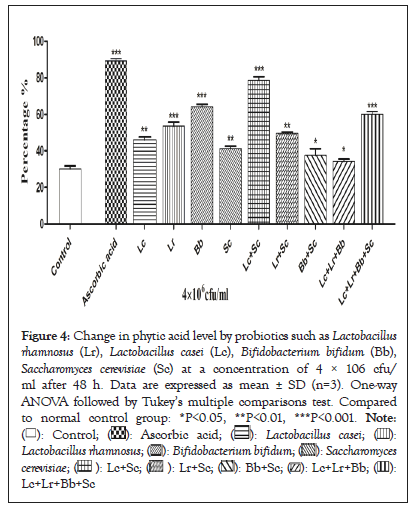
Figure 4: Change in phytic acid level by probiotics such as Lactobacillus rhamnosus (Lr), Lactobacillus casei (Lc), Bifidobacterium bifidum (Bb), Saccharomyces cerevisiae (Sc) at a concentration of 4 × 106 cfu/ml after 48 h. Data are expressed as mean ± SD (n=3). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: *P<0.05, **P<0.01, ***P<0.001.
Amino acid profile
The formation of essential and non-essential amino acids in fermented and unfermented wheat is shown in Figure 5. The formation of amino acids is increased in wheat after fermentation as compared to unfermented wheat (control). The observations that are analysed from this study agreed with the former reports regarding the composition of amino acids in fermented cereals [32]. Increase in essential amount of amino acids was found in different concentrations depending upon the probiotic culture used. The non-essential amino acids recorded were in the range of 0.642 mg/g of proline in wheat fermented by Lactobacillus rhamnosus to 395.38 mg/g of glycine in wheat fermented in Lactobacillus casei in the combination of Saccharomyces cerevisiae and the range of essential amino acids found were 8.67 mg/g of isoleucine in wheat fermented by Lactobacillus rhamnosus to 187.24 mg/g of valine in wheat fermented by Saccharomyces cerevisiae. Essential amino acids such as 16.54 mg/g of threonine and 161 mg/g of valine were found in wheat after fermentation by single culture of Lactobacillus rhamnosus while as 16.84 mg/g of valine and 93.53 mg/g of methionine were recorded in wheat fermented by Lactobacillus casei. The concentration of methionine was higher 135.71 mg/g in wheat fermented by single culture of Saccharomyces cerevisiae while as Bifidobacterium bifidum also showed increased concentration of methionine as 106.10 mg/g and concentration of valine was found to 115.27 mg/g. Methionine plays an important role in metabolism and detoxification, helps in absorption of zinc and selenium minerals that are vital to our body while as valine prevents the breakdown of muscle because it supplies the muscles with an extra glucose that is responsible for energy production during physical activity, thus stimulate muscle growth and regeneration and is involved in energy production [33]. Non-essential amino acids such as aspartic acid in the concentration of 161.89 mg/g was found higher in wheat fermented by combined culture of Lactobacillus rhamnosus, Lactobacillus casei, Bifidobacterium bifidum while as it was recorded as 118.24 mg/g in combination of Lactobacillus rhamnosus and Saccharomyces cerevisiae. These results were in line with those Leitao-Goncalves, et al [34]. Most of the amino acids are mainly found in dairy products, meat, and eggs but are absent in the cereals or are present in very minute quantity. But after fermentation, there is an increase in amino acids in cereals. These amino acids activate the key enzymes in protein synthesis after physical exercise and activate a certain pathway in the body. As it is well known, and is confirmed in the present study fermented products are a good source of sulfur containing amino acids such as methionine and cysteine. Fermentation resulted in the elevation in the yield of essential and non-essential amino acids except arginine. These results were similar with O.U. Eka [35]. Therefore, it can be suggested that fermented wheat can be used as good source for high quality of protein and essential amino acids (Figure 5).
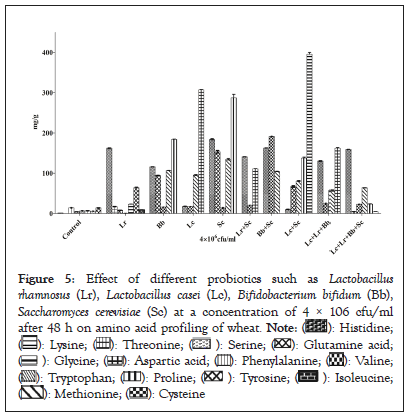
Figure 5: Effect of different probiotics such as Lactobacillus rhamnosus (Lr), Lactobacillus casei (Lc), Bifidobacterium bifidum (Bb), Saccharomyces cerevisiae (Sc) at a concentration of 4 × 106 cfu/ml after 48 h on amino acid profiling of wheat.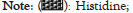

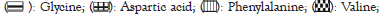
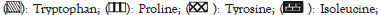
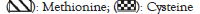
Fermentation of wheat grains with Lactobacillus species and probiotics such as Bifidobacterium bifidum also showed a higher increase in nutritive value mainly amino acid content and protein content. It appears from the results that fermented wheat is high in nutrients particularly in proteins. The current trend in food science is to produce the diets rich in protein and amino acids but with the less content of phytic acids. The present study confirms that fermentation of wheat grains with Lactobacillus species and other different mixed cultures including Bifidobacterium bifidum and Saccharomyces cerevisiae is the potential and effective method in reducing the phytic acid content. As reported in previous studies, the phytic acid decreased to 90% can improve the absorption and bioavailability of different minerals particularly calcium used to maintain the bone health. pH of fermented grains were decreased after fermentation by all the probiotics either by single culture or mixed co-culture, pH being the treatment condition for the reduction of phytic acid by increasing the solubility of phytic acid and activity of phytase. It was also revealed that overall nutritional quality of the fermented wheat is increased by both single and sequential culture of probiotics. Lactobacillus casei with combination of Saccharomyces cerevisiae showed the increased antioxidant activity followed by the single culture of Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus casei. Further study is needed to investigate new metabolites that are formed during the fermentation process and effect of new different probiotics.
The authors acknowledge the senior research fellowship program of the Indian Council Medical Research (ICMR) under the letter No.3/1/2/153/2019-(Nut). Department of Science and Technology, GOI for providing research set up.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Altaf A, Jha B (2023) Evaluating the Impact of Fermentation by Multiple Probiotics on Proximate Composition, Amino Acid Profile, Antioxidant Activity and Dephytinization of Wheat Grains. J Prob Health. 11:311.
Received: 27-Feb-2023, Manuscript No. JPH-23-21267; Editor assigned: 01-Mar-2023, Pre QC No. JPH-23-21267 (PQ); Reviewed: 15-Mar-2023, QC No. JPH-23-21267; Revised: 22-Mar-2023, Manuscript No. JPH-23-21267 (R); Published: 30-Mar-2023 , DOI: 10.35248/2329-8901.23.11.311
Copyright: © 2023 Altaf A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.