Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2015) Volume 6, Issue 4
Micellar electrokinetic chromatography (MEKC) provides a simple and rapid approach for determining n-octanolwater partition coefficients (log Pow). A set of non-hydrogen bonding (NHB), hydrogen bond accepting (HBA) and hydrogen bond donating (HBD) benzene derivatives with known log Pow values was used as sample solutes. Two novel cationic gemini surfactants with different head groups were used as pseudostationary phases. Sodium dodecyl sulfate (SDS) was also used for comparison. Two approaches were applied for the determination of log Pow values: calibration curve and phase ratio. In calibration curve approach, the MEKC retention factors (log k) of six alkyl phenyl ketones were plotted against their literature log Pow values for constructing the calibration curve. Log Pow values of benzene derivatives were then determined from the slope and the y-intercept of the linear calibration line. In the phase ratio approach, total surfactant concentration, critical micelle concentration, partial specific molar volume and experimental log k values were utilized for estimation of the log Pow values. Both approaches provided comparable results for HBA solutes; however, the calibration curve approach and phase ratio approach were found to be more successful for NHB and HBD solutes, respectively. In general, gemini surfactants provided better estimated log Pow values for NHB and HBA solutes while SDS gave better values for HBD solutes.
Keywords: Gemini surfactants; Micellar electrokinetic chromatography; n-Octanol-water partition coefficient; log Pow; Partial specific volume; Phase ratio
CMC: Critical Micelle Concentration; HBA: Hydrogen Bond Accepting; HBD: Hydrogen Bond Donating; NHB: Non-Hydrogen Bonding; log Pow: n-octanol-water Partition Coefficients; MEKC: Micellar Electrokinetic Chromatography; PSV: Partial Specific Volume
Lipophilicity, which correlates with the bioactivity of chemicals, is an important molecular descriptor and its determination constitutes an important element in pharmaceutical characterization of drug candidates since drugs must pass across various biological membranes to reach its site of action. Its determination is of great importance in a variety of fields such as in micellar catalyst, [1] in estimation of the toxic effect of substances in animals and plants, [2] in prediction of chemical adsorption in soil, [3] and in method development and optimization in micellar electrokinetic chromatography (MEKC) [4]. Introduced by Hansch and Fujita for biological activity of chemicals, the logarithm of the partition coefficient between n-octanol and water (log Pow) has been widely used as a general measure of lipophilicity [5-7]. Shake-flask methods is well-known method for determination of log Pow values of chemicals [8,9]. However, this method is cumbersome, time-consuming, needs skilled operator, and requires relatively large amount of pure compounds. After equilibrium between n-octanol and water, the relative concentration of the sample in each layer needs to be determined using spectroscopic or chromatographic techniques. In addition, n-octanol and water system does not mimic the biological model because biomembranes consisting of relatively rigid phospholipids are different from n-octanol-water system in terms of physicochemical property and size.
Due to the drawbacks of the direct measurement of log Pow, alternative methods such as high performance liquid chromatography (HPLC) [10-12] and theoretical calculation methods [13,14] have been introduced. High correlations are observed between the logarithms of retention factors in reversed-phase HPLC (RP-HPLC) and log Pow values [10]. However, due to the excessive retention in RP-HPLC at a purely aqueous mobile phase, the direct measurement of log Pow values is not achievable for many compounds.
As an alternative to RP-HPLC, electrokinetic chromatography (EKC) with micelles [15,16] and microemulsion [17,18] has been introduced as a simple and inexpensive analytical tool for log Pow determination. Owing to its high efficiency and resolving power, requirement for small sample and buffer size, ease and speed of separation, MEKC has been a technique of choice for separation of a variety of charged and neutral compounds since its introduction by Terabe et al. [19] In MEKC, solutes are separated based on their differential partitioning between the aqueous phase and the micellar phase (i.e., pseudostationary phase). One of the major advantages of MEKC over other separation techniques is the feasibility of manipulating the selectivity by simply rinsing the capillary with the solution of a new micellar phase with diverse physicochemical properties [16].
Correlation between micelle-water partition coefficient, log Pmw, and log Pow has been known since 1975 [20]. Good correlations between log Pmw, and log Pow for phthalate esters have been shown in the literature using MEKC [21]. Because the retention factor (log k) in MEKC is directly related to the log Pmw, a linear relationship between log k and log Pow for aromatic solutes in anionic surfactant systems has also been confirmed [22]. In addition to the anionic surfactants, several other surfactant systems such as cationic, anionic-nonionic mixed micelles, [23,24] bile salts, [25] and micro emulsions [26,27] have also been utilized for log Pow determination.
Due to their unique properties, gemini surfactants have been introduced as alternative pseudo stationary phases in MEKC [28-30]. Gemini surfactants are made up of two hydrophobic carbon chains and two polar head groups covalently linked to each other through a spacer. As compared to their single-chain analogues with the same chain length and head group, geminis generally exhibit superior properties [31,32]. They possess remarkably lower critical micelle concentration (CMC), low Krafft point and C20 values (surfactant concentration that reduces the surface tension of the solvent by 20 mNm-1). They have better wetting, solubilizing and foaming properties, closer packing of the hydrophobic groups, and stronger interaction with the oppositely charged surfactants. Head group could be anionic, cationic, zwitterionic and nonionic; spacer can be polar or nonpolar, flexible or rigid, short or long. The nature and length of spacer can have a significant effect on the physicochemical properties and morphology of the gemini aggregates [32].
In present study, two cationic gemini surfactants, 1,1'-didodecyl- 1,1'-but-2-yne-1,4-diyl-bis-pyrrolidinium dibromide (G1) and N,N'- didodecyl-N,N,N',N'-tetramethyl-N,N'-but-2-ynediyl-di-ammonium dibromide (G2) were used as pseudostationary phases in MEKC for Pow determination. Sodium dodecyl sulfate (SDS), a commonly used anionic conventional pseudostationary phase with identical hydrocarbon chain length, was also used for comparison. Both gemini surfactants contain 2-butyne spacer and the same hydrocarbon chain lengths (C12), however, G1 has pyrrolidinium while G2 has dimethyl ammonium head group (chemical structures of surfactant systems are provided in Figure 1). To the best of our knowledge, no other study has yet been reported in the literature using cationic gemini surfactants for Pow determination.
Chemicals
All benzene derivatives, alkyl phenyl ketone (APK) homologues, disodium hydrogenphosphate, sodium dihydrogenphosphate, and sodium hydroxide were obtained from Alfa Aesar (Ward Hill, MA, USA). Deionized water was obtained from a water purification system from Millipore (Milford, MA, USA). SDS was purchased from EMD Chemicals (Gibbstown, NJ, USA). The gemini surfactants G1 and G2 were donated by Professor Fredric M. Menger’s Research Laboratory at Emory University (Atlanta, GA, USA). All chemicals were used as received without any further purification.
Characterization of surfactants
Surface tension measurement was used for CMC determination of the gemini surfactants and SDS. This method is based on the change in surface tension as a factor of surfactant concentration. The surface tension of surfactant solutions with given concentrations were measured at ambient temperature by a KSV Sigma 703D digital tensiometer (Monroe, CT, USA) using a DuNoüy ring. Surface tension values were plotted against surfactant concentration and the CMC value was taken as the breakpoint of the curve. The details of the experiment are explained elsewhere, [29] thus, are not reported here. Partial specific volume, PSV, is defined as the increase in volume upon dissolving 1.0 g of a dry material in a large volume of a solvent at constant temperature and pressure. Since the measurement of such small volume change is nearly impossible, an approach based on density measurement of surfactant solutions was used for determination of PSV. Five solutions with varied surfactant content were prepared in deionized water and their densities were measured at 25°C using a high-precision digital DMA 4500 density meter (Anton Paar, Ashland, VA, USA). The PSV values were obtained from the y-intercept of a graph of reciprocal of density against weight fraction of solvent. Experimental details and calculations on PSV are discussed elsewhere [29] and thus are not repeated here.
Capillary electrophoretic separations
Instrumentation
An Agilent CE system (Agilent Technologies, Palo Alto, CA, USA) equipped with a diode array detector was used for MEKC separations. The system control and data handling were done using 3D-CE ChemStation software. The MEKC separations were performed in fused-silica capillaries (Polymicro Technologies, Tucson, AZ, USA) with dimensions of 66.0 cm total length (57.5 cm effective length) × 50 μm ID (360 μm OD). Capillaries used in this study were cut from the same capillary bundle and were reactivated thoroughly after each surfactant system using deionized water (10 min) and 1.0M NaOH (ca. 20 min) to eliminate possible cross contamination. Each new capillary was activated with 1M NaOH (30 min at 40°C) and deionized water (10 min at 25°C) before use. For a typical MEKC run, the capillary was rinsed for 3 min with triply deionized water and for 3 min 0.1M NaOH followed by 3 min rinse with separation buffer between injections. Each day, the capillary was reactivated by rinsing with 1M NaOH (10 min) and triply deionized water (5 min). Unless otherwise noted, the applied voltage was -30 kV for cationic geminis and +30 kV for anionic SDS. The injection size was 50 mbar for 1 s. Peaks were identified by comparison of their individual UV-spectrum obtained from diode array detector or in case of confusion the individual solute was spiked into the mixture.
Preparation of separation buffers and solute solutions
The background electrolyte (BGE) was prepared by dissolving appropriate amounts of anhydrous NaH2PO4 and anhydrous Na2HPO4 in deionized water to obtain 100 mM solution of each. A stock solution of 10 mM phosphate buffer with pH of 7.0 was prepared from the mixture of 42.3 mL NaH2PO4 and 57.7 mL Na2HPO4. When necessary, dilute HCl or NaOH was used for adjustment of pH. Run buffers were prepared by addition of various amount of surfactant to the BGE. The final concentration of the two geminis in run buffers was 6.0 mM each and that of SDS was 40.0 mM. All run buffers were filtered through a 0.45 μm syringe filter (Nalgene, Rochester, NY, USA) followed by degassing using ultrasonication for about one min before used in MEKC experiments. All stock solutions of the test solutes were prepared in methanol with a concentration of ca. 20 mg/mL each and diluted with 50:50 methanol:deionized water before injection. The final solute concentration ranged from ca. 0.2 to 0.5 mg/mL.
Calculations
The retention factor values, k, of neutral solutes were calculated by use of the following equation [33]:
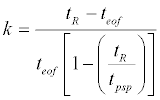 (1)
(1)
Where tR, teof and tpsp are the migration times of solute, EOF, and the pseudostationary phase, respectively. Methanol and undecanophenone were used as teof and tpsp markers, respectively. Solute partition coefficient between bulk aqueous and micellar phase, Pmw, is directly related to k and the phase ratio, β (Equation 2) [4].
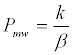 (2)
(2)
The β is defined as the ratio of the volume of micellar phase (Vmc) over that of aqueous phase (Vaq) and is related to the total concentration, Csurf, the partial specific molar volume, V , and the CMC of the surfactant (Equation 3) [33].
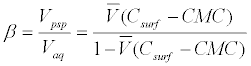 (3)
(3)
The relationship between log k and log Pow may be expressed using the following functional form [34,35]:
log Pow=alog k+b
Where a and b are constants that represent the slope and intercept of a linear calibration line.
Characterization of surfactant systems
The physicochemical properties of the surfactants are listed in Table 1. As compared with SDS, a conventional surfactant with the same carbon chain length, geminis have lower CMC and phase ratio but higher PSV values. The PSV values (in mL·g-1) are converted to partial specific molar volumes, PMV, (in L·mol-1) using molar masses of the surfactants (PMV=PSV × MM × 0.001 L/mL).
| Physicochemical property | Pseudostationary phase | ||
|---|---|---|---|
| G1 | G2 | SDS | |
| Chemical formula | C36H70N2Br2 | C32H66N2Br2 | C12H25NaO4S |
| Molar mass (g mol-1) | 690.76 | 638.69 | 288.38 |
| CMCa* in pure water (mM) | 0.82 | 0.71 | 8.0 |
| CMCa* in 10 mM phosphate buffer (pH 7.0) (mM) | 0.21 | 0.11 | 3.0 |
| Partial specific volumeb*(mL·g-1) | 0.91 | 1.05 | 0.85 |
| Partial specific molar volumec(L·mol-1) | 0.63 | 0.58 | 0.25 |
| Phase ratiod | 0.0037 | 0.0034 | 0.0092 |
*Values from reference [29].
aCritical micelle concentrations were determined in deionized water or 10 mM phosphate buffer (pH 7.0) by surface tensiometer at ambient temperature.
bPartial specific volume was determined in deionized water by density meter at 25°C.
cPartial specific molar volume was calculated using PSV and molar mass of the surfactant (i.e., PMV=PSV × MM × 0.001 L/mL).
dPhase ratio was determined from Equation 3.
Table 1: Physicochemical properties of investigated surfactants.
Estimation of octanol-water partition coefficients using alkyl phenyl ketones calibration curves
Like most other separation techniques, electrophoretic technique requires very small amount of compound (which does not have to be pure), is fast compared to traditional methods used for Pow determination, and are relatively easy to automate. As presented in Equation (4), this method is indirect. In other words, it is based on the construction of a correlation between a retention property characteristic of the solute (e.g., log k) and the separation system for a training set of solutes with known log Pow values. Further measurements of log k in the separation system can be used to estimate log Pow values for other compounds of interest.
Six APKs, i.e., acetophenone, propiophenone, butyrophenone, valerophenone, hexanophenone and heptanophenone, with known log Pow values were selected as training solutes to construct the calibration curve needed for estimation of log Pow values of 29 sample benzene derivatives. The sample benzene derivatives used in this study are characterized as non-hydrogen bond donors (NHBs; 10 solutes), hydrogen bond acceptors (HBAs; 9 solutes), and hydrogen bond donors (HBDs; 10 solutes). The NHB solutes include alkyl- and halo-substituted benzenes and polycyclic aromatic hydrocarbons (e.g., naphthalene) and do not hold any hydrogen bonding functional groups. However, due to the aromatic ring(s), they are considered to be weak hydrogen bond acceptors. The HBAs possess only hydrogen bond accepting functional groups on the aromatic ring, whereas, the HBDs have both hydrogen bond donating and hydrogen bond accepting functional groups. Based on their pKa values, all test solutes are believed to be neutral under experimental conditions.
The six APK training solutes were analyzed and their log k values were determined under the given MEKC conditions. The log k values were then plotted against their literature log Pow values for construction a linear calibration graph (Figure 2). High correlations between log k and log Pow values for ketones were obtained with correlation coefficients (R2) greater than 0.99 in all surfactant systems. Linear regression analysis yielded the following equations:
Figure 2: Plots of log Pow values of 6 alkyl phenyl ketones versus their log k values using gemini (G1, G2) and SDS surfactant systems. MEKC separation conditions: 6.0 mM G1, 6 mM G2, 40.0 mM SDS in 10 mM phosphate buffer (pH 7.0); pressure injection, 50 mbar for 1 s; applied voltage, -30 kV for G1 and G2 and +30 kV for SDS; temperature, 25°C; UV detection at 254 nm. The regression equation for alkyl phenyl ketones in each pseudostationary phase is given in text.
G1 system: log Pow=1.308 logk+2.587, R2=0.993 (5)
G2 system: log Pow=1.419 logk+2.521, R2=0.995 (6)
SDS system: log Pow=1.360 log k+1.953, R2=0.992 (7)
Based on the correlation coefficients obtained, the gemini surfactants provided relatively better linear equations than SDS. The log k values for 29 test solutes were determined under the same MEKC conditions. The estimated log Pow values, or more precisely, the micelle-water partition coefficient, log Pmw, for benzene derivatives were then using the experimental log k values in Equations 5-7. The estimated log Pmw and the differences between estimated log Pmw and log Pow (Δ) values are listed in Table 2. The best estimated log Pmw values were obtained for HBA solutes (including APKs) in all three surfactant systems (Figure 3), as indicated by their smaller Δ values. The absolute mean Δ values are 0.08, 0.06, and 0.18 log units for G1, G2 and SDS surfactant systems, respectively. In log Pow estimation studies, it is important to remember that the structures of the training set and samples must be similar [11]. Since the training ketones show hydrogen bond accepting characteristics, superior log Pmw estimates for HBA samples are not surprising. Furthermore, scientifically sound log Pmw values were determined for NHB solutes, which are weak hydrogen bond acceptors, due to the benzene ring(s) in their structures. The average Δ values for NHB solutes are 0.19, 0.27, and 0.67 log units in G1, G2 and SDS surfactant systems, respectively. Conversely, the poorest estimates were obtained for HBD solutes, especially in gemini surfactant systems, as can be seen from relatively higher average Δ values. This signifies that when structurally unrelated compounds are correlated via Equation (4), incorrect estimates of log Pow values are obtained. This is because separation mechanisms that influence log k are not usually same as those influence log Pow [11]. These results demonstrate G1 and G2 gemini surfactant systems are suitable for the high throughput estimation of log Pow of weakly basic (i.e., HBA) and, to some extent, nonpolar (i.e., NHB) compounds, but not acidic compounds; while SDS system is suitable for log Pow estimation of both HBA and HBD solutes.
| G1 | G2 | SDS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Analytes | log Powa | log Pmw | Δb | log Pmw | Δ | log Pmw | Δ | |
| NHB solutes | |||||||||
| 1 | Benzene | 2.13 | 1.88 | 0.25 | 1.74 | 0.39 | 1.34 | 0.79 | |
| 2 | Toluene | 2.69 | 2.42 | 0.27 | 2.31 | 0.38 | 1.94 | 0.75 | |
| 3 | Chlorobenzene | 2.84 | 2.67 | 0.17 | 2.55 | 0.29 | 2.10 | 0.74 | |
| 4 | Bromobenzene | 2.99 | 2.94 | 0.05 | 2.78 | 0.21 | 2.30 | 0.69 | |
| 5 | Ethylbenzene | 3.15 | 2.90 | 0.25 | 2.81 | 0.34 | 2.45 | 0.70 | |
| 6 | p-Xylene | 3.15 | 2.94 | 0.21 | 2.85 | 0.30 | 2.51 | 0.64 | |
| 7 | 4-Chlorotoluene | 3.33 | 3.19 | 0.14 | 3.11 | 0.22 | 2.69 | 0.64 | |
| 8 | Iodobenzene | 3.25 | 3.29 | 0.04 | 3.15 | 0.10 | 2.63 | 0.62 | |
| 9 | Propylbenzene | 3.68 | 3.46 | 0.22 | 3.38 | 0.30 | 3.05 | 0.63 | |
| 10 | Naphthalene | 3.35 | 3.64 | -0.29 | 3.53 | -0.18 | 2.87 | 0.48 | |
| Absolute mean Δc | 0.19 | 0.27 | 0.67 | ||||||
| HBA solutes | |||||||||
| 11 | Benzonitrile | 1.56 | 1.62 | -0.06 | 1.59 | -0.03 | 1.42 | 0.14 | |
| 12 | Acetophenone | 1.58 | 1.68 | -0.10 | 1.66 | -0.08 | 1.68 | -0.10 | |
| 13 | Nitrobenzene | 1.85 | 1.97 | -0.12 | 1.92 | -0.07 | 1.53 | 0.32 | |
| 14 | Methyl benzoate | 2.16 | 2.13 | 0.03 | 2.13 | 0.03 | 2.07 | 0.09 | |
| 15 | Propiophenone | 2.20 | 2.16 | 0.04 | 2.13 | 0.07 | 2.10 | 0.10 | |
| 16 | 4-Chloroacetophenone | 2.35 | 2.46 | -0.11 | 2.45 | -0.10 | 2.38 | -0.03 | |
| 17 | 4-Nitrotoluene | 2.45 | 2.50 | -0.05 | 2.50 | -0.05 | 2.13 | 0.32 | |
| 18 | Ethyl benzoate | 2.64 | 2.60 | 0.04 | 2.63 | 0.01 | 2.56 | 0.08 | |
| 19 | 4-Chloroanisole | 2.82 | 2.96 | -0.14 | 2.87 | -0.05 | 2.45 | 0.37 | |
| Absolute mean Δc | 0.08 | 0.06 | 0.18 | ||||||
| HBD solutes | |||||||||
| 20 | Benzyl alcohol | 1.08 | 1.42 | -0.34 | 1.38 | -0.30 | 1.03 | 0.05 | |
| 21 | Phenol | 1.49 | 2.31 | -0.82 | 2.32 | -0.83 | 0.98 | 0.51 | |
| 22 | 3-Methylphenol | 1.96 | 2.55 | -0.59 | 2.56 | -0.60 | 1.19 | 0.77 | |
| 23 | 4-Chloroaniline | 1.83 | 2.79 | -0.96 | 2.62 | -0.79 | 1.82 | 0.01 | |
| 24 | 4-Flourophenol | 1.77 | 2.81 | -1.04 | 2.85 | -1.08 | 1.54 | 0.23 | |
| 25 | 4-Ethylphenol | 2.58 | 3.29 | -0.71 | 3.35 | -0.77 | 2.11 | 0.47 | |
| 26 | 4-Chlorophenol | 2.35 | 3.43 | -1.08 | 3.47 | -1.12 | 1.90 | 0.45 | |
| 27 | 3-Chlorophenol | 2.49 | 3.53 | -1.04 | 3.59 | -1.10 | 1.88 | 0.61 | |
| 28 | 4-Bromophenol | 2.59 | 3.71 | -1.12 | 3.73 | -1.14 | 2.12 | 0.47 | |
| 29 | 3-Bromophenol | 2.63 | 3.80 | -1.17 | 3.85 | -1.22 | 2.09 | 0.54 | |
| Absolute mean Δc | 0.89 | 0.89 | 0.41 | ||||||
| Alkyl phenyl ketones | |||||||||
| 30 | Butyrophenone | 2.73 | 2.64 | 0.09 | 2.65 | 0.08 | 2.64 | 0.09 | |
| 31 | Valerophenone | 3.26 | 3.17 | 0.09 | 3.20 | -0.06 | 3.18 | 0.08 | |
| 32 | Hexanophenone | 3.79 | 3.77 | 0.02 | 3.79 | 0.00 | 3.77 | 0.02 | |
| 33 | Heptanophenone | 4.32 | 4.42 | -0.10 | 4.39 | -0.07 | 4.42 | -0.10 | |
| Absolute mean Δc | 0.07 | 0.06 | 0.08 | ||||||
| Overall absolute mean Δd | 0.31 | 0.32 | 0.33 | ||||||
aLiterature values. From reference [16].
bΔ=log Pow(Lit)-log Pmw(MEKC)
cAbsolute mean of differences for each subset solutes.
dAbsolute mean of differences for all solutes
Table 2: Estimated log Pmw values obtained from calibration curves using six alkyl phenyl ketones as training solutes.
Estimated log Pmw values versus literature log Pow values plots show apparent differences between the two values (Figure 4). The divergence is due to the fact that the nature of the interactions between soluteoctanol and solute-surfactant systems is different. As seen in Figure 4, three distinct congeneric lines can be observed for sample solutes using the three pseudostationary phases. This shows that the factors that influence retention in G1, G2 and SDS surfactant systems are notably different from those that influence octanol-water partitioning. Figure 4 also suggests that nonpolar NHB solutes have stronger interaction with octanol whereas polar HBD solutes tend to interact more with G1 and G2 surfactants. In addition, octanol and geminis are found to have similar affinities for polar HBA solutes while SDS has relatively higher affinity for the same solutes. It is also important to note that the slope of the regression line for HBA solutes is close to unity in all three surfactant systems, suggesting that these surfactants and octanol-water systems possess very similar partitioning mechanisms for these solutes. Also, unlike G1 and G2 geminis, SDS and octanol-water systems have similar partitioning mechanism for HBD solutes.
Figure 4: Plots of log Pow values versus estimated log Pmw values determined from calibration curves using A) G1, B) G2, and C) SDS surfactant systems. MEKC separation conditions are the same as Figure 2. Dashed lines represent the trend line for NHB, HBA and HBD subset solutes and solid line represents the trend line for all sets together. The regression equations for each subset and complete solutes are provided on the right side of the plots.
To better understand the origins of the congeneric behavior for surfactant systems studied here, it is helpful to compare the linear solvation energies (LSER) results discussed in our previous report [29]. It has been shown in the literature that polarizability and volume of solute enhance the log Pow, while dipolarity and hydrogen bond accepting ability of solute inhibit it. The solute hydrogen bond donating ability, however, has been found to have no significant influence on log Pow values [16,36,37]. The geminis, SDS and octanol-water systems show large positive values, indicating that the cohesive energy density and dispersion interaction term has a great amount of influence in MEKC retention. The magnitude of coefficients v suggests that hydrophobic solutes prefer to interact more with octanol and SDS systems. It is suggested by Abraham et al. that the relative values of coefficients are more descriptive than their absolute values [37]. Similar ratios indicate similar interactions between solutes and pseudostationary phase (or octanol phase). Since hydrophobic interaction has the major influence on partitioning, the e, s, a, and b coefficients have been normalized against v coefficient for G1, G2 and SDS systems in this study (Table 3). For comparison, normalized values for octanol-water system and the differences between the ratios were also included. The results in Table 3 show that the b/v ratios for G1 and G2 as well as e/v for G2 are practically similar to that for octanol-water system and the remaining ratios are somehow different. The major difference is in a/v ratios of G1 (0.33) and G2 (0.35) as well as in b/v ratio of SDS (0.38) seem to be the sources of the observed congeneric behavior and different lines in Figure 4.
| Surfactant system | System constant ratios | ||||
|---|---|---|---|---|---|
| v | e/v | s/v | a/v | b/v | |
| 1,1'-didodecyl-1,1'-but-2-yne-1,4-diyl-bis-pyrrolidinium dibromide (G1) | 3.10 | 0.19 | -0.07 | 0.35 | -0.97 |
| N,N'-didodecyl-N,N,N',N'-tetramethyl-N,N'-but-2-ynediyl-di-ammonium dibromide (G2) | 3.05 | 0.13 | -0.03 | 0.37 | -0.92 |
| Sodium dodecyl sulfate (SDS) | 3.16 | 0.04 | 0.00 | -0.06 | -0.56 |
| Octanol–water partition coefficient (log Pow) | 4.07 | 0.11 | -0.22 | 0.02 | -0.94 |
| Differences between ratios | |||||
| G1 and log Pow | 0.97 | 0.08 | 0.15 | 0.33 | 0.03 |
| G2 and log Pow | 1.02 | 0.02 | 0.19 | 0.35 | 0.02 |
| SDS and log Pow | 0.91 | 0.15 | 0.22 | 0.08 | 0.38 |
*LSER coefficients obtained from geminis and SDS are taken from reference [29]. Coefficients for octanol-water system were calculated using MS Excel (n=29).
Table 3: LSER coefficient ratios for surfactant systems and octanol-water system*.
Estimation of octanol-water partition coefficients using phase ratios
To minimize the errors due to the application of the training ketones, a new approach was applied to the estimation of log Pow values. As mentioned earlier, Pmw is directly related to k and the phase ratio (Equation 2). Retention factor, k, can be easily obtained in any chromatographic technique; however, the phase ratio cannot be measured accurately in conventional (e.g., liquid) chromatography due to the fact that the phase ratio varies from column to column and even with time for a given column [4]. Thus, the use of Equation 2 for estimation of log Pac in conventional chromatography is not feasible. However, since the pseudostationary phase remains constant under given experimental conditions and the physicochemical properties of the micellar phase do not depend on the capillary system and separation column, unlike conventional chromatographic methods, MEKC can be used for log Pac estimations using the phase ratio. Since k is directly related to solute partition between the bulk aqueous buffer solution and the micellar phase, Equation 2 is applicable in MEKC. As stated in Equation 3, the phase ratio is related to total surfactant concentration, critical micelle concentration, and partial specific molar volume of the surfactant. Since it is a characteristic of the micellar phase, the phase ratio remains constant at a given MEKC conditions. Unlike HPLC, it does not vary from capillary to capillary or with time. Before using Equation 2 for log Pow estimations, the phase ratio was determined first by using the total surfactant concentrations (6.0 × 10-3 mol·L-1 geminis and 4.0 × 10-2 mol·L-1 SDS), CMC values of surfactants under experimental conditions (2.1 × 10-4 mol·L-1 G1, 1.1 × 10-4 mol·L-1 G2 and 3.0 × 10-3 mol·L-1 SDS) and partial specific molar volume values listed in Table 1.
The estimated log Pmw obtained from the phase ratio equation and the differences between estimated log Pmw and log Pow (Δ) values are listed in Table 4. As seen in Figure 5, the estimated log Pmw values are very comparable with those obtained from alkyl phenyl ketones calibration curves (Figure 3). The absolute mean Δ values for HBA solutes (0.10 log units for both G1 and G2 systems and 0.17 log units for SDS) show the feasibility of the phase ratio approach for log Pmc estimations. As compared with calibration curve approach, slightly poorer estimates were obtained for NHB (Δ values are 0.35, 0.38, and 0.70 log units for G1, G2 and SDS, respectively) and APK solutes. It is worth mentioning that significant improvements were observed for HBD solutes. Similar to the calibration curves, estimated log Pmw values obtained from phase ratio calculations are plotted against the literature log Pow values (Figure 6). Based on the correlation coefficient values, the estimated log Pmw results were found to be slightly better than the previous values. These preliminary estimates can be further improved by careful determination of phase ratios and partial specific molar volumes.
| G1 | G2 | SDS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Analytes | log Powa | log Pmw | ∆b | log Pmw | ∆ | log Pmw | ∆ | |
| NHB solutes | |||||||||
| 1 | Benzene | 2.13 | 1.91 | 0.22 | 1.91 | 0.22 | 1.59 | 0.54 | |
| 2 | Toluene | 2.69 | 2.32 | 0.37 | 2.32 | 0.37 | 2.03 | 0.66 | |
| 3 | Chlorobenzene | 2.84 | 2.51 | 0.33 | 2.49 | 0.35 | 2.14 | 0.70 | |
| 4 | Bromobenzene | 2.99 | 2.71 | 0.28 | 2.65 | 0.34 | 2.29 | 0.70 | |
| 5 | Ethylbenzene | 3.15 | 2.69 | 0.46 | 2.67 | 0.48 | 2.40 | 0.75 | |
| 6 | p-Xylene | 3.15 | 2.71 | 0.44 | 2.70 | 0.45 | 2.45 | 0.70 | |
| 7 | 4-Chlorotoluene | 3.33 | 2.90 | 0.43 | 2.88 | 0.45 | 2.58 | 0.75 | |
| 8 | Iodobenzene | 3.25 | 2.98 | 0.27 | 2.91 | 0.34 | 2.53 | 0.72 | |
| 9 | Propylbenzene | 3.68 | 3.11 | 0.57 | 3.07 | 0.61 | 2.84 | 0.84 | |
| 10 | Naphthalene | 3.35 | 3.24 | 0.11 | 3.17 | 0.18 | 2.71 | 0.64 | |
| Absolute mean ∆c | 0.35 | 0.38 | 0.70 | ||||||
| HBA solutes | |||||||||
| 11 | Benzonitrile | 1.56 | 1.71 | -0.15 | 1.81 | -0.25 | 1.65 | -0.09 | |
| 12 | Acetophenone | 1.58 | 1.76 | -0.18 | 1.86 | -0.28 | 1.83 | -0.25 | |
| 13 | Nitrobenzene | 1.85 | 1.98 | -0.13 | 2.04 | -0.19 | 1.72 | 0.13 | |
| 14 | Methyl benzoate | 2.16 | 2.10 | 0.06 | 2.19 | -0.03 | 2.12 | 0.04 | |
| 15 | Propiophenone | 2.20 | 2.12 | 0.08 | 2.19 | 0.01 | 2.14 | 0.06 | |
| 16 | 4-Chloroacetophenone | 2.35 | 2.35 | 0.00 | 2.41 | -0.06 | 2.35 | 0.00 | |
| 17 | 4-Nitrotoluene | 2.45 | 2.38 | 0.07 | 2.45 | 0.00 | 2.17 | 0.28 | |
| 18 | Ethyl benzoate | 2.64 | 2.46 | 0.18 | 2.54 | 0.10 | 2.48 | 0.16 | |
| 19 | 4-Chloroanisole | 2.82 | 2.73 | 0.09 | 2.71 | 0.11 | 2.40 | 0.42 | |
| Absolute mean ∆c | 0.10 | 0.10 | 0.17 | ||||||
| HBD solutes | |||||||||
| 20 | Benzyl alcohol | 1.08 | 1.56 | -0.48 | 1.66 | -0.58 | 1.36 | -0.28 | |
| 21 | Phenol | 1.49 | 2.24 | -0.75 | 2.32 | -0.83 | 1.32 | -0.17 | |
| 22 | 3-Methylphenol | 1.96 | 2.42 | -0.46 | 2.49 | -0.53 | 1.48 | -0.48 | |
| 23 | 4-Chloroaniline | 1.83 | 2.60 | -0.77 | 2.53 | -0.70 | 1.94 | -0.11 | |
| 24 | 4-Flourophenol | 1.77 | 2.61 | -0.84 | 2.69 | -0.92 | 1.73 | 0.04 | |
| 25 | 4-Ethylphenol | 2.58 | 2.98 | -0.40 | 3.05 | -0.47 | 2.15 | 0.43 | |
| 26 | 4-Chlorophenol | 2.35 | 3.08 | -0.73 | 3.13 | -0.78 | 2.00 | 0.35 | |
| 27 | 3-Chlorophenol | 2.49 | 3.16 | -0.67 | 3.21 | -0.72 | 1.98 | 0.51 | |
| 28 | 4-Bromophenol | 2.59 | 3.29 | -0.70 | 3.31 | -0.72 | 2.16 | 0.43 | |
| 29 | 3-Bromophenol | 2.63 | 3.37 | -0.74 | 3.40 | -0.77 | 2.14 | 0.49 | |
| Absolute mean ∆c | 0.65 | 0.70 | 0.33 | ||||||
| Alkyl phenyl ketones | |||||||||
| 30 | Butyrophenone | 2.73 | 2.49 | -0.24 | 2.56 | -0.17 | 2.54 | -0.19 | |
| 31 | Valerophenone | 3.26 | 2.89 | -0.37 | 2.94 | -0.32 | 2.94 | -0.32 | |
| 32 | Hexanophenone | 3.79 | 3.34 | -0.45 | 3.36 | -0.43 | 3.37 | -0.42 | |
| 33 | Heptanophenone | 4.32 | 3.83 | -0.49 | 3.78 | -0.54 | 3.85 | -0.47 | |
| Absolute mean ∆c | 0.30 | 0.30 | 0.28 | ||||||
| Overall absolute mean ∆d | 0.35 | 0.37 | 0.37 | ||||||
aLiterature values. From reference [16].
bΔ=log Pow(Lit)- log Pmw(MEKC)
cAbsolute mean of differences for each subset solutes.
dAbsolute mean of differences for all solutes.
Table 4: Estimated log Pmw values obtained from phase ratios.
Figure 6: Plots of log Pow values versus estimated log Pmw values determined from phase ratio approach using A) G1, B) G2, and C) SDS surfactant systems. MEKC separation conditions are the same as Figure 2. Dashed lines represent the trendline for NHB, HBA and HBD subset solutes and solid line represents the trendline for all sets together. The regression equations for each subset and complete solutes are provided on the right side of the plots.
Two cationic gemini surfactants, 1,1'-didodecyl-1,1'-but-2-yne-1,4- diyl-bis-pyrrolidinium dibromide (G1) and N,N'-didodecyl-N,N,N',N'- tetramethyl-N,N'-but-2-ynediyl-di-ammonium dibromide (G2) were used as pseudostationary phases in MEKC for Pmw determinations. Sodium dodecyl sulfate (SDS) was also used for comparison. Both gemini surfactants contain 2-butyne spacer with twelve hydrocarbon chain length. However, G1 has pyrrolidinium but G2 has dimethyl ammonium head groups. MEKC was successfully applied as a simple and rapid approach for determining log Pow using two novel gemini surfactants and SDS. Two approaches were applied for determination of log Pow values: calibration curve and phase ratio. In calibration curve approach, the log k values of six alkyl phenyl ketones were plotted against their literature log Pow values for constructing linear calibration curve. Log Pow values of 29 sample benzene derivatives were then determined from the slope and the y-intercept of the calibration line. In the phase ratio approach, total surfactant concentration, critical micelle concentration, partial specific molar volume and experimental log k values were utilized for estimation of the log Pow values. Both approaches were found to provide very comparable results. In general, gemini surfactants provided better estimated log Pow values for NHB and HBA solutes while SDS gave better values for HBD solutes.
This work was supported by a Support of Competitive Research (SCORE) Program grant from the National Institute of General Medical Sciences, one of the National Institutes of Health (Grant No. S06GM078246). The authors acknowledge the financial support of the Fayetteville State University Research Initiative for Scientific Enhancement (FSU-RISE) Program. The authors are thankful to Dr. Menger’s Research Team at Emory University (Atlanta, Georgia) for donating the cationic gemini surfactants.