
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)Volume 10, Issue 5
Introduction: Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) are acute phase reactants and
Gamma-Glutamyl Transpeptidase (GGT) is a liver enzyme that is associated with prognosis in patients with
Hepatocellular Carcinoma (HCC).
Objective: To evaluate the value of ESR and GGT singly and together in HCC prognosis and as predictors of tumor
aggressiveness parameters.
Methods: The database from a large cohort of Turkish HCC patients was examined retrospectively for the prognostic
usefulness of blood ESR and GGT levels and the associated patient subgroup characteristics.
Results: Patients with low vs. high blood ESR or GGT values had greater than double survival, with Hazard Ratios
(HR) by Cox regression of 1.543 and 1.833 respectively. The combination of ESR plus GGT was associated with a 3-
fold survival difference and an HR of 2.410. Patients with high vs. low ESR plus GGT levels had significantly greater
maximum tumor diameters, alpha-fetoprotein levels, multifocality and percent of patients with portal vein
thrombosis. Significant survival differences were also found for patients with low serum alpha-fetoprotein levels.
Addition of CRP levels to the ESR plus GGT combination added further discriminant survival information, but for
greater computational complexity.
Conclusions: ESR plus GGT is a useful and powerful prognosticator in HCC patients, including those with low
alpha-fetoprotein levels and significantly associates with all the tumor parameters of HCC patients.
HCC; CRP; ESR; PVT; MTD
HCC: Hepatocellular Carcinoma; PVT: Portal Vein Thrombosis; AFP: Alpha-Fetoprotein; GGTP: Gamma Glutamyl Transpeptidase; ALKP: Alkaline Phosphatase; CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate; MTD: Maximum Tumor Diameter; CT: Computerized Axial Tomography; MRI: Magnetic Resonance Imaging
Indices of inflammatory have been long recognized to be both part of the carcinogenic process [1-5] as well as useful prognosticators for many cancers. Serum C-Reactive Protein (CRP) and blood Erythrocyte Sedimentation Rate (ESR) are amongst the best studied inflammation-associated parameters for many diseases [6-8] and CRP has been shown to be prognostically useful for many cancers, including HCC [9-15]. Recently, ESR has also attracted attention for its use in HCC prognostication [16-18]. Furthermore, serum levels of the liver enzyme, Gamma-Glutamyl Transpeptidase (GGT) has been recognized to be associated with HCC [19-22] and an HCCspecific isozyme (GGT-II) has been characterized and studied [23-30]. In the present work, the 3 inflammation markers CRP, ESR and GGT were examined in relation to HCC patient survival and the combination of ESR and GGT was found to be superior to the single parameters and was associated with higher levels of HCC aggressiveness factors of Maximum Tumor Diameter (MTD), Alpha-Fetoprotein (AFP), multiple tumor nodules and percent of patients with macroscopic Portal Vein Thrombosis (PVT).
Patient data
A database was retrospectively analyzed of 573 non-transplant HCC patients who had both survival data and baseline tumor parameter data, including CT scan information on HCC maximum Tumor Diameter (MTD), number of tumor nodules and presence or absence of macroscopic portal Vein Thrombosis (PVT); blood erythrocyte sedimentation rate values; serum Alpha-Fetoprotein (AFP) levels; complete blood count; routine blood liver function tests, (total bilirubin, GGTP, ALKP, albumin, transaminases), as well as patient demographics. Diagnosis was made either via tumor biopsy or according to international AASLD guidelines. Database management conformed to legislation on privacy and this study conforms to the ethical guidelines of the Declaration of Helsinki and approval for this retrospective study on de-identified HCC patients was obtained by the Institutional Review Board of each participating institution, as previously reported (REF).
Statistical analysis
Continuous variables were summarized by median, first quartile (25th percentile) and third quartile (75th percentile). Comparisons between two groups were performed by Mann Whitney U test. Categorical variables were expressed as count and percentage, comparisons according to these variables were made by Pearson’s chi-square, and continuity corrected chisquare or Fisher’s exact test where appropriate. Kaplan-Meier method and Log-Rank test were used for survival analysis. Cox regression was used for Hazard Ratio (HR) estimations. For all analyses, two-tailed significance level was considered as 0.05. IBM SPSS Statistics for Windows version 22.0 (NY, USA) was used for statistical analysis.
Survival of HCC patients according to blood GGT or ESR levels, alone or in combination
A survival analysis was calculated by the Kaplan-Meier method for the 3 single parameters, serum GGT, serum CRP and blood ESR levels, each dichotomized according to cutoffs that were previously found (REFS). Patients with low GGT, ESR or CRP levels lived significantly longer that patients with high GGT, ESR or CRP levels (Table 1A). Hazard ratios (HRs) were significantly greater for high versus low GGT (HR 1.543, p=0.002), for high versus low ESR (HR 1.833, P=0.001) and for high versus low CRP levels (HR 1.570, p<0.001) by univariate Cox regression analysis. But by multivariate Cox regression analysis, only CRP and ESR HRs were significant in Figure 1A&1B.
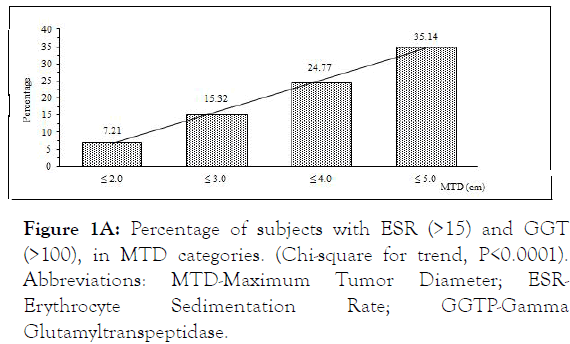
Figure 1A: Percentage of subjects with ESR (>15) and GGT (>100), in MTD categories. (Chi-square for trend, P<0.0001). Abbreviations: MTD-Maximum Tumor Diameter; ESRErythrocyte Sedimentation Rate; GGTP-Gamma Glutamyltranspeptidase.
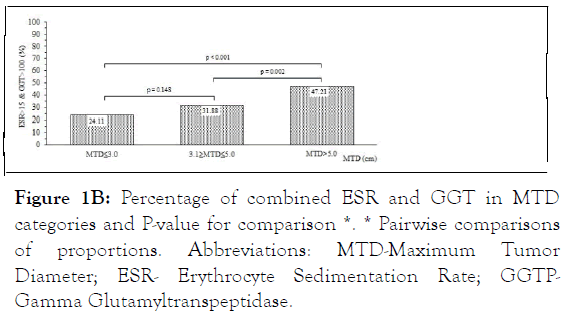
Figure 1B: Percentage of combined ESR and GGT in MTD categories and P-value for comparison *. * Pairwise comparisons of proportions. Abbreviations: MTD-Maximum Tumor Diameter; ESR- Erythrocyte Sedimentation Rate; GGTPGamma Glutamyltranspeptidase.
| Kaplan-Meier | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Analysis | Cox regression | Cox regression | |||||
| Survival time (mo) | Log-Rank | HR | HR | HR | HR | ||
| Mean ± SE | P-value | (95% CI) | P-value | (95% CI) | P-value | ||
| Total | GGT ≤ 100 | 88.52 ± 6.64 | 0.002 | reference | reference | ||
| cohort | GGT >100) | 41.72 ± 4.20 | 1.543 (1.167-2.041 | 0.002 | 1.292 (0.916-1.823) | 0.144 | |
| Total | ESR ≤ 15 | 101.81 ± 11.03 | 0.001 | reference | reference | ||
| cohort | ESR>15 | 42.40 ± 3.36 | 1.833 (1.286-2.611) | 0.001 | 1.506 (1.022-2.219) | 0.039 | |
| Total | CRP ≤ 6 | 72.26 ± 6.12 | <0.001 | reference | reference | ||
| cohort | CRP>6 | 38.45 ± 3.96 | 1.570 (1.229-2.005) | <0.001 | 1.843 (1.287-2.641) | 0.001 | |
Abbreviations: GGT-Gamma-Glutamyl Transpeptidase (IU/mL); ESR-Erythrocyte Sedimentation Rate (mm/hour); CRP-C-Reactive Protein (mg/dL); mo-Months0.009.
Table 1A: Kaplan-Meier analysis for HCC patient survival according to blood GGT, ESR and CRP alone.
ESR has previously been combined with CRP levels, to show a modest increase in survival compared with each parameter alone (REF). We next examined the combination of GGT with CRP, and the combination of GGT with ESR, and then compared the survival of patients having high or low levels of each combination (Table 1B). We found that survival by Kaplan- Meier analysis of patients with low levels of combination GGT plus CRP was significantly longer than the survival of patients with high levels of combination GGT plus CRP (91.75 vs. 43.82 months, p=0.002) and the HR by Cox regression analysis was 1.792, P=0.005. The survival of patients with low levels of combination GGT plus ESR was also significantly longer than the survival of patients with high levels of combination GGT plus ESR (115.43 vs. 39.25 months, P<0.001) and the HR was 2.410, P=0.001. Since the HR was much higher for the GGT plus ESR combinations, we separately examined that combination in patients with either small <5 cm MTD tumors or with large >5 cm MTD tumors. We found that survival was significantly longer in patients with low compared to high combination GGT plus ESR levels, regardless of whether they had small or large tumors in Table 1B, lower half.
| Kaplan-Meier | Univariate | ||||
|---|---|---|---|---|---|
| Analysis | Cox regression | ||||
| Survival time (mo) | Log-Rank | HR | HR | ||
| Mean ± SE | P-value | (95% CI) | P-value | ||
| Total | GGT ≤ 100 & CRP ≤ 6 | 91.75 ± 7.33 | 0.004 | reference | |
| cohort | GGT>100 & CRP>6 | 43.82 ± 7.24 | 1.792 (1.191-2.696) | 0.005 | |
| Total | GGT ≤ 100 & ESR ≤ 15 | 115.43 ± 12.13 | <0.001 | reference | |
| cohort | GGT>100 & ESR>15 | 39.25 ± 5.29 | 2.410 (1.456-3.990) | 0.001 | |
| MTD<5 cm | GGT ≤ 100 & ESR ≤ 15 | 84.03 ± 9.85 | 0.032 | reference | |
| GGT>100 & ESR>15 | 43.61 ± 7.20 | 2.324 (1.045-5.169) | 0.039 | ||
| MTD≥5 cm | GGT ≤ 100 & ESR ≤ 15 | 68.42 ± 10.46 | 0.004 | reference | |
| GGT>100 & ESR>15 | 24.86 ± 3.52 | 2.799 (1.339-5.853) | 0.006 | ||
Table 1B: Kaplan-Meier analysis for HCC patient survival according to blood GGT plus ESR or CRP in combination.
Blood and tumor characteristics of patients with low or high levels of combination blood GGT plus ESR levels
The clinical blood level characteristics of patients with high versus low GGT plus ESR combination levels were then compared. For high combination GGT plus ESR levels, patients had significantly lower Hb, albumin and HDL levels, but higher numbers of platelets and higher levels of all liver function tests than patients with lower levels of combination GGT plus ESR in Table 2A.
| GGT ≤ 100 & ESR ≤ 15 | GGT>100 and ESR>15 | ||
|---|---|---|---|
| Median (1stQ-3rdQ) | Median (1stQ-3rdQ) | P | |
| Hb, g/dL | 13.4(12-15) | 11.9(10.6-13.1) | <0.001 |
| Platelets,103/μL | 138.5(75-215) | 170(106.8-247.3) | 0.018 |
| Albumin, g/dL | 3.6(2.9-3.8) | 2.6(2.2-3.1) | <0.001 |
| HDL, mg/dL | 36.3(29.2-44.6) | 28.5(17.7-38) | 0.005 |
| LDL, mg/dL | 84(66.2-109.5) | 97(74.3-119.5) | 0.139 |
| CRP, mg/dL | 0.9(0.3-4.8) | 4.6(1.5-10) | <0.001 |
| ALKP, IU/mL | 94(66-116.5) | 192(135-300) | <0.001 |
| AST, IU/mL | 42(30-63) | 88.5(51.8-146) | <0.001 |
| T.BİL, mg/dL | 1(0.8-1.6) | 1.5(0.9-2.9) | <0.001 |
| Cholesterol, mg/dL | 1.2(1-1.3) | 1.2(1.1-1.4) | 0.058 |
| ALT, IU/mL | 36(23.5-58.5) | 52.5(32-98) | <0.001 |
*1stQuartile is the 25th percentile - 3rdQuartile is the 75th percentile. Abbreviations: GGT: Gamma-Glutamyl Transpeptidase (IU/mL); ESR: Erythrocyte Sedimentation Rate (mm/hour); CRP: C-Reactive Protein; ALB: Albumin (g/dL); ALT: Alanine Aminotransferase (IU/L); ALKP: Alkaline Phosphatase (IU/mL); T. BIL; Total Bilirubin (mg/dL); HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein.
Table 2A: Blood parameters of patients with high or low blood GGTP plus ESR levels.
The tumor characteristics were then compared. MTD, AFP, patients with tumor multifocality and percent of patients with PVT were all found to be elevated in patients with high versus low combination GGT plus ESR levels in Table 2B.
| GGTP ≤ 100 & ESR ≤ 50 | GGTP>100 and ESR>50 | ||
|---|---|---|---|
| Median (1stQ-3rdQ) | Median (1stQ-3rdQ) | P | |
| MTD size | 5.1 (3.5-7.5) | 7.5 (4.5-10.6) | 0.008 |
| AFP | 15.6 (6.2-417.5) | 239 (19.5-1210) | <0.001 |
| % | % | P | |
| Tumor # ≤ 2 | 71 | 44.3 | <0.001 |
| Tumor # >2 | 29 | 55.7 | |
| PVT (-) | 84.9 | 61 | 0.001 |
| PVT (+) | 15.1 | 39 | |
Abbreviations: GGT-Gamma-Glutamyl Transpeptidase (IU/mL); ESR-Erythrocyte Sedimentation Rate (mm/hour); AFP-Alpha-Fetoprotein (IU/mL); MTD-Maximum Tumor Diameter; PVT-Macroscopic Portal Vein Thrombosis.
Table 2B: Tumor characteristics in patients with high or low blood GGTP plus ESR levels.
Survival, blood and tumor characteristics in patients with low serum AFP levels
Survival was analyzed according to the Kaplan-Meier method. Patients with low levels of the GGT plus ESR combination had significantly longer survival than patients with high levels of the GGT plus ESR combination (134.69 vs. 38.58 mo, P<0.001) and an HR by Cox regression of 3.745; P=0.001. The low AFP patients with low combination GGT plus ESR levels had longer survival than patients with low combination GGT plus ESR in the total cohort of Table 1. Patients with low AFP levels and either small <5 cm MTD or large >5 cm MTD, were then separately analyzed for survival. Patients with small tumors had significantly longer survival in the low compared to the high combination GGT plus ESR group (96.26 vs. 44.69 mo, P=0.016) and an HR by Cox of 3.886, P=0.023. Patients with larger tumors had almost significant differences in their survival in the 2 combination groups, P=0.054, but the HR by Cox regression was not significant.
The pattern of differences in the blood parameters was nearly identical to that seen in the total cohort as shown in Table 2A, except that platelet differences had lost significance in the low AFP cohort in Table 3A.
| Kaplan-Meier | Univariate | ||||
|---|---|---|---|---|---|
| Analysis | Cox regression | ||||
| Survival time (mo) | Log-Rank | HR | HR | ||
| Mean ± SE | p-value | (95% CI) | P-value | ||
| Total | GGT ≤ 100 & ESR ≤ 15 | 134.69 ± 13.91 | <0.001 | reference | |
| sub-cohort | GGT>100 & ESR>15 | 38.58 ± 6.62 | 3.745 (1.722-8.146) | 0.001 | |
| MTD<5 cm | GGT ≤ 100 & ESR ≤ 15 | 96.26 ± 10.39 | 0.016 | reference | |
| GGT>100 & ESR>15 | 44.69 ± 9.28 | 3.887 (1.207-12.512) | 0.023 | ||
| MTD ≥ 5 cm | GGT ≤ 100 & ESR ≤ 15 | 70.58 ± 12.16 | 0.054 | reference | |
| GGT>100 & ESR>15 | 24.78 ± 4.03 | 2.886 (0.930-8.957) | 0.067 | ||
GGT: Gamma-Glutamyl Transpeptidase (IU/mL); ESR: Erythrocyte Sedimentation Rate (mm/hour); AFP: Alpha-Feto Protein; Mo-Months
Table 3A: Kaplan-Meier analysis for patients with low serum AFP (<100 IU/mL) levels.
| GGT ≤ 100 and ESR ≤ 15 | GGT>100 and ESR>15 | ||
|---|---|---|---|
| Median (1stQ-3rdQ.) | Median (1stQ-3rdQ.) | P | |
| Hb | 13,8 (12-15.1) | 12 (10.9-13.1) | <0.001 |
| Platelets | 138 (86-216) | 153 (88-253.5) | 0.267 |
| Albumin | 3.6 (3-3.9) | 2.7 (2.3-3.1) | <0.001 |
| HDL | 36.3 (30.8-44.2) | 29.3 (20.6-38) | 0.051 |
| LDL | 84 (65.5-113) | 97 (85-114) | 0.138 |
| CRP | 0.5 (0.3-2.5) | 4.8 (1.6-11) | <0.001 |
| ALKP | 87.5 (61.5-116) | 204 (141-305.3) | <0.001 |
| AST | 35.5 (29-57) | 78 (48-145.5) | <0.001 |
| T.BİL | 1 (0.8-1.3) | 1.4 (0.9-2.7) | 0.003 |
| Cholesterol | 1.2 (1-1.3) | 1.2 (1.1-1.4) | 0.264 |
| ALT | 35.5 (25-56.3) | 57 (32.5-99.5) | 0.001 |
*1stQuartile is the 25th percentile-3rdQuartile is the 75th percentile
Abbreviations and units, are as in Table 2.
Table 3B: Blood parameters of patients with low AFP (<100 IU/mL) and high or low GGTP plus ESR levels.
When the tumor characteristics of patients with high versus low combination GGT plus ESR were compared (Table 3C), AFP differences were unsurprisingly lost in this low AFP sub-cohort of patients, as were MTD differences, as all patients in the low AFP sub-cohort had smaller tumors than in the total cohort shown in Table 2B. However, there were significantly more patients with both tumor multifocality and PVT, in the patient group with high levels of the GGT plus ESR combination, than in the group with low levels of combination GGT plus ESR in Table 3C.
| GGTP ≤ 100 and ESR ≤ 15 | GGTP>100 and ESR>15 | ||
|---|---|---|---|
| Median (1stQ-3rdQ.) | Median (1stQ-3rdQ.) | P | |
| MTD size | 5 (3-7) | 5 (3.3-8.9) | 0.27 |
| AFP | 7.8 (3.4-13.3) | 11.5 (2.5-36.8) | 0.256 |
| % | % | p | |
| Tumor # ≤ 2 | 81.8 | 43.9 | <0.001 |
| Tumor # > 2 | 18.2 | 56.1 | |
| PVT (-) | 95.6 | 71.9 | 0.004 |
| PVT (+) | 4.4 | 28.1 |
GGT: Gamma-Glutamyl Transpeptidase (IU/mL); ESR: Erythrocyte Sedimentation Rate (mm/hour); AFP: Alpha-Feto Protein; MTD: Maximum Tumor Diameter (cm); PVT: Macroscopic Portal Vein Thrombosis.
Table 3C: Tumor characteristics in patients with high or low serum GGTP plus ESR levels, for low AFP (<100 IU/mL) patients.
Triplet combination of blood GGT, ESR plus CRP levels
Since CRP has already been established as a prognostically useful inflammatory marker [6-11], CRP levels were added to GGT plus ESR doublet, to form a triplet marker combination. Survival was examined for patients who had low or high levels of triplet combination GGT, ESR plus CRP (Table 4). For the total cohort (Table 4A), survival for low vs. high triplet combination levels was 116.3 vs. 32 months, P<0.001 with an HR by Cox of 3.585, P<0.001. This was slightly better than the performance of the GGT plus ESR doublet results in Table 1B. For the low AFP sub-cohort of patients, survival for low vs. high triplet combination levels was 135.18 vs. 16.22 months, P<0.001 and HR by Cox of 5.756, P=0.002. This HR was higher than the performance of doublet GGT plus ESR results, HR of 3.745 in Table 3, for the identical low AFP patients.
| Kaplan-Meier Analysis | Univariate Cox regression | |||
|---|---|---|---|---|
| Log-Rank | HR | HR | ||
| P-value | (95% CI) | P-value | ||
| Total cohort: GGT ≤ 100 & ESR ≤ 15 & CRP ≤ 6 | <0.001 | reference | ||
| GGT>100 and ESR>15 and CRP>6 | 3.585 (1.806-7.116) | <0.001 | ||
| Lo AFP patients* | GGT ≤ 100 and ESR ≤ 15 and CRP ≤ 6 | <0.001 | reference | |
| GGT>100 and ESR>15 and CRP>6 | 5.756 (1.946-17.028) | 0.002 | ||
*Low AFP patients, AFP<100 IU/mL.
Abbreviations: GGT: Gamma-Glutamyl Transpeptidase (IU/mL); ESR: Erythrocyte Sedimentation Rate (mm/hour); CRP: C-Reactive Protein (mg/dL); AFP: Alpha-Fetoprotein.
Table 4A: Kaplan-Meier analysis and Cox regression according to blood GGT, ESR and CRP values together in total and low serum AFP cohorts.
Tumor characteristics were next examined according to the triplet combination (Table 4B). MTD of 9.7 cm was significantly greater than MTD of 5 cm, for the high vs. low levels of the triplet combination, P<0.001, and the range was greater than for the duplet combination of 7.5 vs. 5.1 cm shown in Table 2, P=0.008. For the other tumor parameters, the differences were comparable for high vs. low triplet combination as for high vs. low doublet combination. The triplet combination was finally used to examine the tumor characteristics in the low AFP patients (Table 4C). Patients with high triplet values had significantly larger tumors than those with low triplet values, MTD 5 vs. 11 cm, P=0.001, whereas there were no differences in MTD for the high vs. low doublet values seen in Table 3C. When tumor multifocality and percent of patients with PVT were examined, there were significantly higher percentages in the high value triplet group when compared to the low value triplet group (Table 4C, lower half), and this result was similar for the doublet combination of Table 3.
| GGTP ≤ 100 and ESR ≤ 15 C and RP ≤ 6 | GGTP>100 and ESR>15 and CRP>6 | ||
|---|---|---|---|
| Median (1stQ-3rdQ.) | Median (1stQ-3rdQ.) | P | |
| MTD size | 5 (3-7) | 9.7 (6.7-14) | <0.001 |
| AFP | 12.8 (5.6-185.7) | 456 (25.8-2969) | 0.001 |
| % | % | P | |
| Tumor # ≤ 2 | 84.1 | 48.5 | 0.002 |
| Tumor #>2 | 15.9 | 51.5 | |
| PVT (-) | 95.7 | 52.9 | <0.001 |
| PVT (+) | 4.3 | 47.1 |
Abbreviations: GGT: Gamma-Glutamyl Transpeptidase (IU/mL); ESR: Erythrocyte Sedimentation Rate (mm/hour); AFP: Alpha-Fetoprotein; MTD: Maximum Tumor Diameter (cm); PVT: Macroscopic Portal Vein Thrombosis.
Table 4B: Tumor characteristics in patients with high or low blood GGT, ESR and CRP levels together in total cohort.
| GGTP ≤ 100 and ESR ≤ 15 and CRP ≤ 6 | GGTP>100 and ESR>15 and CRP>6 | ||
|---|---|---|---|
| Median (1stQ-3rdQ.) | Median (1stQ-3rdQ.) | P | |
| MTD size | 5(3-7) | 11(6.6-14.5) | 0.001 |
| AFP | 7.765(4-15.6) | 15.15(2.99-41) | 0.276 |
| % | % | p | |
| Tumor # ≤ 2 | 93.5 | 54.5 | 0.009 |
| Tumor #>2 | 6.5 | 45.5 | |
| PVT (-) | 96.9 | 54.5 | 0.003 |
| PVT (+) | 3.1 | 45.5 |
Abbreviations: GGT: Gamma-Glutamyl Transpeptidase (IU/mL); ESR: Erythrocyte Sedimentation Rate (mm/hour); AFP: Alpha-Fetoprotein (<100IU/mL); MTD: Maximum Tumor Diameter (cm); PVT: Macroscopic Portal Vein Thrombosis.
Table 4C: Tumor characteristics in patients with high or low blood GGT, ESR and CRP levels together in low serum AFP cohort.
The prognosis for most solid tumors of adults is typically much better when treatment can be delivered early in the course of the disease, instead of later. This usually means threatening at a smaller tumor stage, which in turn depends on early diagnosis. HCC usually does not cause symptoms till late in the disease, so the best current approach for early diagnosis is through screening of asymptomatic patients who are at risk for HCC development. This is most practically done for patients who are already known to have cirrhosis, which is a pre-malignant state and is most commonly caused by chronic infection with hepatitis B or C or alcoholism. Current guidelines recommend screening by use of ultrasonography, typically at 6 monthly intervals in those at risk. A blood test would be more convenient, but the historically-used AFP has recently fallen out of favor, due to the lack of increased AFP levels in at least 50% of HCC patients and to the often-low levels in patients with smaller tumors-just where a surveillance test is most needed. Hence the search for newer screening tools. Prognosticating blood tests have similar problems in that AFP, when it is elevated is very useful, although it is not elevated in many patients. Since HCC arises mainly on the basis of chronic viral or toxic (alcohol or metabolic) liver inflammation, recent attention has focused on inflammatory biomarkers as predictors of survival. Currently, the Glasgow Index, a combination of serum albumin and CRP levels has been found to be useful in many studies [31-37], as have various ratios of platelets to lymphocytes (PLR), neutrophils to lymphocytes (NLR), monocytes to lymphocytes [12-15,38], and more recently, blood CRP levels, ESR levels [16-18] and GGT levels [19-33].
We chose the combination of serum GGT plus blood ESR levels, since on comparison with single parameters ESR, CRP and GGT and double parameters of these same indices, the combination of GGT and ESR had the largest spread of survival times and the highest HR values (Tables 1A and 1B) and cutoffs were selected as published previously for this patient cohort [18,19,39]. Interestingly, when high vs. low values of the combination of GGT plus ESR were compared, there were significant different differences in survival by Kaplan-Meier analysis and Cox regression, when patients with either small or large tumors were examined (Table 1B, lower half). The blood parameter characteristics of patients having high or low levels of combination GGT plus ESR were significantly different for almost every parameter that was studied (Table 2A), with especially higher levels of the liver function tests and CRP and lower (worse) albumin levels for the high GGT plus ESR group. This same group also had significantly worse measures of every tumor parameter (size, number, AFP values and percent of patients with PVT), as shown in Table 2B. A similar approach was used for the low-AFP patient subcohort (Table 3). Survival was also significantly shorter for patients having high vs. low GGT plus ESR combination (less than 50% time), and the Kaplan-Meier analysis Table 3A) showed significant survival differences for the total cohort and the patient group with small tumors (P<0.001 and P=0.016) and approached significance in patients with larger tumors (P=0.054). The high vs. low blood GGT plus ESR groups also showed significant differences in most of the clinical blood parameters (Table 3B), but the tumor parameters were only significantly different for the analysis of percent of patients with tumor multifocality or PVT (Table 3C). The 2 groups were not different in respect of MTD (likely because low serum AFP patients have smaller tumors) nor in respect of AFP levels (by definition of low AFP patients). The poorer survival in the combination with higher GGT plus ESR levels must thus have been due to either worse liver function (higher levels of serum bilirubin, AST, ALT and ALKP) or more aggressive tumor biology (as shown in tumor multifocality and PVT), despite the absence of elevated serum AFP levels. The data thus implies that mediators of aggressive HCC biology must exist that are not reflected in AFP levels.
Since CRP levels were previously established as prognostically useful, CRP levels were added to the GGT plus ESR doublet, forming a triplet marker combination. Patients with low levels of the triplet combination had a 3-fold longer survival than those with high levels, both by Kaplan-Meier and Cox regression. For the patients with low serum AFP, the survival advantage for low levels of the triplet combination was almost 8-fold (Table 4A). Similarly for the tumor characteristics. All the tumor parameters were significantly worse in patients with high vs. low triplet combination levels and the same was also true for low serum AFP patients (except serum AFP levels). Thus, comparing the survival values (HRs) for high vs. low values of the doublet and the triplet combinations (Tables 1B vs. 4A), the HRs by Cox regression were 2.3 vs. 3.585, showing that the triplet had more prognostic power, but at a higher level or operational complexity. Similarly for the low serum AFP subcohort of patients (Tables 3A vs. 4A), the HRs were 3.745 vs. 5.756 by Cox regression, for the doublet vs. the triplet combinations. The triplet combination for both the total cohort and the low serum AFP subcohorts showed some superior prognostic ability compared to the doublet combination, but for additional computational complexity.
What might be the explanation for the prognostic power of the GGT plus ESR combination? Both are regarded as inflammation markers. Inflammation has been shown to be related to tumor growth [1-3,5], likely through the release of complex cascades of tumor growth factors, chemokines, cytokines and prostaglandins. GGT is produced by the liver and by HCCs, unlike ESR, and has been regarded as an HCC biomarker [19-30]. It is important in its involvement in cell damage by reactive oxygen species [41] and its levels rise during oxidative stress. Thus, GGT is a monitor of local inflammation and ESR is a monitor of systemic inflammation. Another aspect of their importance is their prognostic value in the large percent of HCC patients with low serum AFP and their possible use in surveillance in patients with known chronic hepatitis B or C. This prognostic value also draws attention to the potential use of anti-inflammatory agents as potential HCC therapies.
This work was supported in part by NIH grant CA 82723 (B.I.C)
Author contributions and acknowledgements: BIC-concept and ideas.
BIC, HA-writing; HB, VG, RD-statistics; UK, KY, NE, AO, EA, HY, HS, AU, AB, SK, OU, YU, BG and AD- data collection, database formation and quality of data evaluation from original source documents.
This work complies with the guidelines of the World Medical Association, Declaration of Helsinki. This work was approved by each institution’s IRB as documented in the methods section.
Citation: Carr B, Akkiz H, Bag HG, Guerra V, Donghia R, Yalçın K, et al. (2020) Erythrocyte Sedimentation Rate and Gamma-glutamyl Transpeptidase Combined are Potent Predictors of Survival and Tumor Characteristics in Hepatocellular Carcinoma Patients. J Clin Trials. 10:425.
Received: 04-Aug-2020 Accepted: 18-Aug-2020 Published: 25-Aug-2020
Copyright: © 2020 Carr B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.