Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2014) Volume 5, Issue 6
Experimental studies were conducted to investigate the photodegradation of diazepam and sertraline, two of the most frequently used psychiatric drugs, induced by xenon lamp irradiation, which overlap the sunlight spectra, and natural sunlight. Degradation kinetics was established indicating the occurrence of autocatalytic reactions. The application of liquid chromatography coupled to quadrupole time-of-flight mass spectrometry allowed for accurate mass measurements and proper fragmentation of target compounds enabling structure elucidation of various photoproducts of diazepam and sertraline formed during the forced photolysis. As a result, phototransformation pathways were proposed including the compounds and routes of degradation described here for the first time. In the final step, the presence of parent and identified compounds was examined in various environmental water samples. Although, the analytes were non-detected it was assumed that they may undergo different process in the environment such as adsorption, dilution, advection or rapid photodegradation of all compounds.
Keywords: Pharmaceuticals; Photolysis; Aqueous environment; Transformation products; Degradation kinetics
DIZ: Diazepam; LC-HRMS: Liquid Chromatography-High Resolution Mass Spectrometry; MeOH: Methanol; PhAC: Pharmaceutically Active Compound; SER: Sertraline; SPE: Solid Phase Extraction; SSRI: Serotonin Reuptake Inhibitor; TP: Transformation Product; UHPLC-QTOF-MS: Ultra-High Pressure Liquid Chromatography–Quadrupole Time-of-Flight-Mass Spectrometry; WTP: Water Treatment Plant; WWTP: Waste Water Treatment Plant
Pharmaceutically active compounds (PhACs) are considered as emerging contaminants, which are not regulated, and have raised a great concern in recent years. The major contributors of these pollutants into the water bodies are wastewater treatment plants (WWTPs), where PhACs are not effectively removed during conventional treatment process [1-3]. It is not unambiguously stated what adverse effects PhACs can cause once they reach natural waters. However, it is suspected that such phenomena as increased bacterial drug resistance [4] genotoxicity [5] or endocrine disruption [6,7] origin from chronic exposure to low doses of PhACs present in aquatic environment.
While various techniques of wastewater treatment or drinking water disinfection are applied, many transformation products (TPs) may be formed. TPs can have the same impact on human health and the environment as the parent pharmaceutical compound; however, they can also be more or less toxic depending on the transformation pathway (possessing or losing the toxicophores). It is also feasible, that the transformation product will receive ‘new’ toxicophore, thus the toxicity of the compound will increase [8]. In this context, it is important to investigate the transformation pathway of various pharmaceuticals in order to track their level and estimate their influence on the environment and living organisms.
Many processes such as sorption, biodegradation, volatilization and photolysis may contribute to PhACs degradation [9]. Due to the pseudo-persistence of many drugs, biochemical reactions have a limited share in the removal of such pollutants [10,11]. It is suspected that in the superficial layer of water bodies photolysis is a main process responsible for the transformation of PhACs [12,13]. In direct photolysis the pollutant, which contain aromatic rings, heteroatom and other functional groups in the structure, can absorb a photon leading to its degradation. In indirect photolysis, the degradation occurs via reaction with active molecules e.g. •OH, 1O2, ROO•, 3DOM*, eaq (generated by photosensitizers such as dissolved organic matter) [9,14,15].
The present work focuses on the photodegradation of two selected psychiatric drugs, sertraline and diazepam. Sertraline is an anti-depressant classified as a second generation serotonin reuptake inhibitor (SSRIs). It is also widely used in the treatment of depression, panic disorders, social phobia, obesity and obsessive-compulsive disorders [16-18]. Diazepam is the most frequently prescribed benzodiazepine used in the treatment of anxiety, insomnia, seizures and alcohol withdrawal, and is commonly known as Valium [19]. The use of psychiatric medications and anti-depressants is rapidly increasing and they become the most widely distributed psychiatric drugs in the world. Therefore, these types of pharmaceuticals are of a great concern since their occurrence in the aquatic environment has been reported in various studies [20-23].
Considering that residues of these drugs have been found in both water bodies and aquatic organisms, the aim of this work was to perform forced degradation under the xenon lamp irradiation in order to identify photoproducts of diazepam and sertraline in water samples. For this purpose liquid chromatography - high resolution mass spectrometry (LC-HRMS) was applied due to its accurate mass measurements, possibility to obtain the isotope pattern and the fragmentation pathway of selected compound. Due to these advantages of this technique the presented results are of a high accuracy and certainty. Furthermore, degradation kinetics in various matrices have been investigated. In the final step different water samples were analyzed for the presence of the parent compounds and their photoproducts. To the best of our knowledge this is the most extensive study on diazepam and sertraline photo-degradation induced by xenon lamp,which simulates natural process occurring in the environment. The performed experiments enabled proposing photodegradation pathways and certain transformations are reported here for the first time.
Sample collection and preparation
Influent and effluent water samples from municipal wastewater treatment plant (WWTP) were collected in Gda?sk, Poland. The wastewater treatment in the facility was based on activated sludge process with nitrification and partial denitrification. Treated and untreated water samples from drinking water treatment plant (WTP) were collected in Gda?sk, Poland. The facility employed partial chlorination and ozonation during water treatment processes. River water samples were collected from two rivers (Radunia and Kacza) exposed to anthropogenic activity located in Pomerania area. The average pH were 6.9 for wastewater influent, 7.5 for wastewater effluent, 7.4 for untreated water, 8.2 for treated water and 7.9 for river water. All kinds of samples for the analysis of the presence of DIZ, SER and their photoproducts were collected in 5 different months (from February to June) and were stored in the dark at 4oC prior to the analysis.
Water samples were prepared using solid phase extraction (SPE) with the application of Oasis HLB cartridges (500 mg, 6 mL; Waters). Briefly, SPE cartridges were washed with MeOH and ultrapure water. Then, a certain volume of water sample containing 1 mL 0.1 M EDTA/100 mL sample was loaded on the sorbent at a flow rate of 5 mL/ min. After the sorbent was dried, the analytes were eluted with 6mL of MeOH. The extracts were evaporated to dryness in a stream of nitrogen and dry residues were redissolved in 1 mL of MeOH/H2O (1:9, v/v). 5 μL of the final extract was injected to UHPLC-QTOF-MS system. Detailed procedure has been previously described [24].
Reagents
Analytical standards of diazepam (DIZ) and sertraline (SER) were purchased from LGC Standards (?omianki, Poland) and Sigma-Aldrich (Pozna?, Poland). LC-MS grade methanol (MeOH) was purchased from Sigma-Aldrich (Pozna?, Poland). Formic acid was from Merck (Warsaw, Poland). Ultrapure water was obtained from a HLP5 system (Hydrolab, Poland). Individual stock solutions of DIZ and SER at concentration level of 1 g/L were prepared in methanol and stored at -80oC.
Irradiation experiments
Degradation kinetic studies of DIZ and SER (at concentration level of 1 mg/L) were investigated in two types of conditions: (1) under simulated solar radiation using xenon lamp and (2) under natural solar radiation. Xe-lamp emits concentrated and constant light spectrum which range overlaps the range of the solar irradiation, and allows performing the experiment independently from the external factor, e.g. weather conditions. While exposing the samples on the solar irradiation it is possible to simulate conditions similar to those in the natural environment.
Solar irradiation experiments were performed for eight different samples: wastewater influent and effluent, untreated and treated water, river water, methanol, ultrapure water for pH 3 and pH 10. The extracts were analyzed by UHPLC-QTOF-MS system every two weeks until the decrease of the concentration below 10% of the initial value.
Xenon lamp irradiation experiments of river water samples containing DIZ or SER (separate experiments) were performed using a small-scale system consisting of a cylindrical photoreactor (25 mL), cooling system, external light system and optical filters. The temperature of the samples during the experiment was maintained at 10°C. The equipment was previously applied for identification of phototransformation products of other pharmaceuticals in river water samples [24,25] and by Górska et al. [26] for photodegradation of phenol.
Identification of photodegradation products in river water samples was performed during the Xe-lamp exposure in order to observe only the photoproducts formed during the irradiation and reducing the possibility ofthe occurrence of other processes (e.g. hydrolysis or biodegradation)to a minimum.
In case of the samples exposed to Xe-lamp light, around 500 μL of a sample was collected during the experiment every 0.5 h (SER) or 1 h (DIZ) until the total degradation of the parent compound. The irradiation by xenon lamp lasted for 4 h (SER) and 18 h (DIZ) that is, until the decrease of the parent analyte concentration below 5%. Each extract was filtered through a 0.45μm nylon syringe filter (Rockwood, USA) and analyzed immediately by UHPLC-QTOF-MS system for further data evaluation.
Analytical methods
The UHPLC-QTOF-MS analysis were carried out using an Agilent 1290 UHPLC system coupled to a hybrid quadrupole time-of-flight (QTOF) mass spectrometer (Agilent 6540 Series Accurate Mass QTOFMS) with Dual ESI interface operated in positive ion mode. Detailed chromatographic conditions and mass spectrometer parameters were previously described in our publication [24]. Briefly, the separation of analytes was performed on XTerra MS C18 column (30 mm×2.1 mm; 3.5μm) maintained at 22°C. The mobile phase A was ultrapure water with 0.05% FA and B was MeOH and the flow rate was set to 0.4 ml/ min. The analysis were carried out in gradient elution (starting from 95% A to 0% A in 15 min, back to 95% A in 1 min and kept at 95% A for 5 min) and the injection volume was 5μL. The QTOF-MS conditions were as follows: sheath gas temperature 400oC at the flow rate of 12 L/ min, capillary voltage 4000 V, nebulizer pressure 20 psig, drying gas 10 L/min, gas temperature 325oC, skimmer voltage 45 V, octopole RF peak 750 V and fragment or voltage 100 V. Analysis were performed in two different modes, MS/MS or TargetMS/MS with various collision energies (10, 20 and 30 V) and the masses were scanned from 50 to 1000 m/z. The instrument was working in the 4GHz high-resolution mode with the acquisition rate of 1.5 spectra/s. Acquisition data were processed using Agilent Mass Hunter Workstation software.
Degradation kinetics
Degradation kinetics were estimated for two types of experiments: (1) during the exposure to xenon lamp radiation, and (2) during the exposure to solar radiation in order to simulate natural processes occurring in the environment. Both experiments lasted until the decrease of DIZ and SER concentration below 10% of the initial value. Based on the values of the coefficient of determination (R2) the degradation kinetics in all samples followed a pseudo-first-order kinetic and the results are presented in Table 1. The time-based pseudofirst- order rate constant (k) was calculated according to the Eq. (1):
| Matrix | Solar irradiation | ||||
| DIZ | SER | ||||
| k (day-1) | t1/2(day) | k (day-1) | t1/2(day) | ||
| Wastewater influent | 0.018 | 38.3 | 0.100 | 6.9 | |
| Wastewater effluent | 0.007a | 102.1a [85]b | 0.140 | 4.9 | |
| River water | 0.007a | 94.5a [85]b | 0.100 | 6.9 | |
| Untreated water | 0.007a | 94.5a [85]b | 0.067 | 10.3 | |
| Treated water | 0.007a | 94.4a [85]b | 0.041a | 16.8a [14]b | |
| Methanol | 0.009a | 72.9 | 0.005a | 129.0a [120]b | |
| Ultrapure water pH 3 | 0.158 | 4.4 | 0.005a | 127.5a [120]b | |
| Ultrapure water pH 10 | 0.009a | 73.5a [49]b | 0.071 | 9.7 | |
| River water | Xe-lamp irradiation | ||||
| k (min-1) | t1/2(min) | k (min-1) | t1/2(min) | ||
| 0.001 | 540.2 | 0.009 | 76.5 | ||
aautocatalytic reaction
b ‘delay time’ duration
Table 1: Photodegradation rate constants (k) and half-lives (t1/2) of DIZ and SER in various matrices exposed to solar or Xe-lamp irradiation.
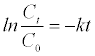 (1)
(1)
where Ct is the concentration of the analyte at the specific irradiation time t and C0 is the initial concentration of the compound.
Half-life t1/2 was calculated using Eq. (2) which was derived by transforming Eq. (1) and replacing Ct with C0/2:
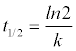 (2)
(2)
The photodegradation rate constants k and the half-lives t1/2for xenon lamp experiment were 0.001 min-1and 9 h for DIZ, and 0.009 min-1 and1.3 h for SER.
The photodegradation kinetics of DIZ and SER induced by solar radiation was investigated in eight different matrices: wastewater influent and effluent, untreated and treated water, river water, methanol, ultrapure water for pH 3 and pH 10 in order to estimate the influence of the type of the matrix and the sample pH on the degradation rate of selected compounds.
Autocatalytic degradation reactions of DIZ and SER have been observed in certain matrices. In such case the initial concentration of the compounds is constant (so-called. delay time) until the specific degradation product is formed in a certain amount that triggers the degradation process running consecutively as the first-order kinetic reaction. An example of such autocatalytic reaction is presented in Figure 1. However, autocatalytic reactions did not occur for all analyzed matrices and was strictly dependent on the compound, the conditions and complexity of the sample composition. The autocatalytic effect of diazepam was observed in all samples, except for wastewater effluent which matrix has a very complex composition, and water of acidic pH (~3), which in this case, significantly accelerates the degradation process. For sertraline this effect was observed in samples with matrices containing a small amount of interfering substances (with a simpler composition), which means that the formation of desired transformation products occurs later than in case of other samples and significantly affects the initiation of the sertraline degradation. The half-life times t1/2 presented in Table 1 are a sum of ‘delay time’ (given in square brackets) and real degradation time. As it can be noticed, the ‘delay time’ is quite long for most of the matrices were it was observed and after it was finished the degradation proceeded rapidly comparatively to the conditions were autocatalysis was not observed.
The photodegradation rate constants k varied from 0.007 to 0.158 day-1 for DIZ and from 0.005 to 0.140 day-1 for SER. The half-lives t1/2 were in range from 4.4 (pH 3) to 102.1 days (wastewater influent) for DIZ and from 4.9 (wastewater influent) to 129 days (methanol) for SER. These results indicate various behaviors of these two psychiatric drugs in considered matrices which may be related to their structures and the presence of different functional groups.
Photodegradation of selected pharmaceuticals
Identification of photodegradation products of DIZ and SER was performed during the exposition of the water extract to Xe-lamp light. The advantage of this approach was independence of various external factors which may negatively affect the experiment. Furthermore, xenon lamp emits the light in the range 250-1000 nm which overlaps the spectrum of the light reaching the Earth. Both direct and indirect photolysis may occur simultaneously due to the constant radiation provided by the lamp.
In order to identify photoproducts of DIZ and SER, samples collected at various time intervals were analyzed by UHPLC-QTOF-MS. Preliminary identification of the probable photoproducts was based on accurate mass measurements of selected (pseudo)molecular ions with a mass error below 5 ppm. Further accurate MS/MS measurements (mass error below 10 ppm) were performed in order to obtain characteristic fragmentation ions necessary to elucidate the structures of suspected photoproducts and the transformation pathways of diazepam and sertraline.
The irradiation of psychiatric drugs solutions resulted in the formation of several chromatographic peaks corresponding to 9 and 7 new compounds for DIZ and SER, respectively (Figure 2a,b).Detailed results are presented in the next sections.
Identification of photoproducts of DIZ: Phototransformation of the widely used benzodiazepine pharmaceuticals diazepam under simulated sunlight in water has been investigated. A total of nine photoproducts, including benzophenones, acridinones and quinazolinones or quinazolines have been identified. All photoproducts, with only two exceptions, have lower molecular weight than the parent compound and photo-induced dimerization was not observed. On the basis of the identified photoproducts (Table 2) the degradation pathway of diazepam has been proposed, and schematically presented in Figure 3.
| Name | Structure | Retention time, min | Molecular formula | [M+H]+theoret. | [M+H]+experim. | Error, ppm | Fragment ions m/z (relative intensity) | |
|---|---|---|---|---|---|---|---|---|
| Diazepam (DIZ) | 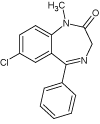 |
8.6 | C16H13ClN2O | 285.0789 | 285.0791 | -0.7 | 285 (100), 154 (25), 257 (19), 193 (17), 222 (17), 228 (15), 182 (5) | |
| DEGRADATION PRODUCTS | 1 | 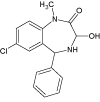 |
4.5 | C16H15ClN2O2 | 303.0895 | 303.0880 | 4.9 | 167 (100), 239 (72), 285 (40), 303 (36) |
| 2 | 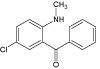 |
10.3 | C14H12ClNO | 246.0680 | 246.0690 | -4.1 | 246 (100), 195 (50), 228 (42), 177 (40), 149 (37) | |
| 3 | 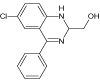 |
4.2 | C15H13ClN2O | 273.0789 | 273.0777 | 4.4 | 158 (100), 273 (80), 196 (13), 228 (12) | |
| 4 | 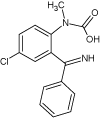 |
7.6 | C15H13ClN2O2 | 289.0738 | 289.0736 | 0.7 | 289 (100), 156 (27), 260 (19), 223 (17), 230 (17), 193 (15) | |
| 5 | 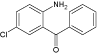 |
8.9 | C13H10ClNO | 232.0524 | 232.0533 | -3.9 | 232 (100), 129(48), 158 (44), 117 (40) 214 (36), 191 (32), 154 (28), 141 (28) | |
| 6 | 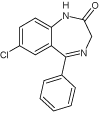 |
6.9 | C15H11ClN2O | 271.0632 | 271.0645 | -4.8 | 271 (100), 140 (88), 165 (56), 208 (50), 243 (18), 193 (13), 226 (9) | |
| 7 | 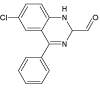 |
7.6 | C15H11ClN2O | 271.0632 | 271.0638 | -2.2 | ||
| 8 | 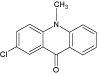 |
8.1 | C14H10ClNO | 244.0524 | 244.0535 | -4.5 | 153 (100), 209 (90), 177 (52) | |
| 9 | 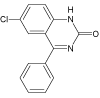 |
7.4 | C14H9ClN2O | 257.0476 | 257.0488 | -4.7 | 207 (100), 124 (530, 223 (20) | |
Table 2: Degradation products of DIZ identified during the irradiation of river water samples by Xe-lamp light.
Xe-lamp irradiation of Diazepam solution caused after 20 minutes formation of two by-products of its degradation (DIZ 1 and DIZ 2). The photoproduct with the m/z 303.0880 may correspond to monohydroxylated derivative of diazepam molecule followed by addition of two hydrogen atoms (reduction of imine fragment). The product DIZ 1 was completely degraded in 180 minutes by photolytic irradiation. The structure of this compound was confirmed by the MS/MS spectrum obtained during the fragmentation of DIZ 1 (Figure 4). There are three characteristic fragment ions of m/z 285.0770, 239.0379 and 167.0368 which correspond to the loss of specific groups as -H2O (formation of diazepam protonated molecule), -C2H6O2 and -C8H8O2, respectively. The formation of this photoproduct was not reported in the literature before.
The second product DIZ 2 (m/z 246.0690) was identified as the 5-chloro-2-methylaminobenzophenone by comparison with the mass spectra of an authentic standard of 5-chloro-2-methylaminobenzophenone known in the literature and reported by West et al. [19]. This compound has also been identified during the exposure of methanolic solutions of diazepam to UV radiation [27]. The structure of this compound was elucidated on the basis of fragmentation pattern obtain during the LC-QTOF-MS analysis in MS/MS mode, which follows by the formation of radicals and it was previously noticed by West et. al. [19].
Continued irradiation (40 min) of Diazepam water solution revealed formation of another degradation product (DIZ 3, m/z 273.0777). Based on its characteristic mass spectra it has been identified as (6-chloro-4- phenyl-1,2-dihydroquinazolin-2-yl)methanol-photoproduct already known in the literature [19]. Photodegradation product DIZ 4 with m/z 289.0736, appeared after 150 minutes of Xe-lamp irradiation of Diazepam. Its formation probably resulted from the opening of the diazepinone seven-membered ring followed by a rearrangement into [4-chloro-2-(phenyl carbonoimidoyl) phenyl] methylcarbamic acid as. This photoproduct is also known in the literature and recent has been reported by West et al.[19].
Xe-lamp irradiation of diazepam solution caused after 90 minutes formation of another by-product of its degradation (DIZ 5). This product (m/z 232.0533) is 15 mass units smaller than the compound DIZ 2 suggesting demethylation of aminomethyl group (elimination of methyl group). The two next products (DIZ 6 and DIZ 7, m/z 271.0645) of diazepam degradation have been identified after 240 minutes of exposure to light. The retention times of these products were different, whereas their mass spectra are identical. Both products’ mass spectra with the most important fragment m/z 270 showed that their mass is 14 units lower, suggesting loss of methylene group from diazepam molecule. The structure of DIZ 6 may correspond to the nordiazepam molecule which is the human metabolite of the diazepam, whereas the structure of DIZ 7, based on fragmentation ions and the reported data [19], was tentatively identified as 6-chloro-4-phenyl-1,2- dihydroquinazoline-2-carbaldehyde.
Continued irradiation of diazepam water solution (300 min) revealed formation of another degradation product (DIZ 8, m/z 244.0535). This compound was assumed to be the result of the opening of the diazepinone seven-membered ring followed by a decarbonylation and rearrangement into a highly stabilized six-membered ring - 2-chloro- 10-methylacridin-9(10H)-one. Note, that this first photodegradation product (DIZ 8) is already known in the literature [19].
The appearance of compound DIZ 9 (m/z 257.0488) after 600 minutes of Xe-lamp exposure shows that the byproduct DIZ 7 is probably oxidized during irradiation and converted into 6-chloro-4- phenylquinazolin-2(1H)-one. Formation of this product is confirmed based on the fragmentation ion (m/z 223.0885) which may correspond to phenylquinazolin-2(1H)-one after elimination of chlorine. This compound is already known in the literature as the product of photodegradation of oxazepam [27].
The irradiation of DIZ was terminated after 18 h when no signal from DIZ was registered and other transformation products were not observed. Further examples of tentative compound identifications for diazepam and its photoproducts DIZ 3 – DIZ 9 based on their mass spectra together with proposed structures of fragment ions during obtained UHPLC-QTOF-MS analysis are provided in the electronic supplementary information (Figures S1−S8).
Photodegradation products of SER: The identified compounds during the photodegradation experiment are summarized in Table 3 and the proposed transformation pathway of sertraline during the photolysis process is presented in Figure 5. As it can be noticed, the process starts by hydroxyl radical attack at the aromatic rings of sertraline molecule, that is much easier then hydroxylation of nonaromatic ring, forming two hypothetical isomers SER 1a and SER 1b (m/z 322.0760). Unfortunately, the obtained information during the UHPLC-QTOF-MS analysis was not sufficient to establish the exact position of the hydroxyl substituent at the aryl ring. Similar products have been reported in the literature [18], however, the suggested hydroxylation occurred in non-aromatic ring which was confirmed during the fragmentation of SER 1a,b at various collision energies no ions (Figure S10).
| Name | Structure | Retention time, min | Molecular formula | [M+H]+ theoret. | [M+H]+ experim. | Error, ppm | Fragment ions m/z (relative intensity) | |
|---|---|---|---|---|---|---|---|---|
| Sertraline (SER) | 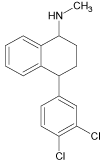 |
6.7 | C17H17Cl2N | 306.0811 | 306.0815 | -1.3 | 159 (100), 275 (55), 129 (45) | |
| DEGRADATION PRODUCTS | 1a | 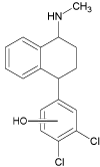 |
4.8 | C17H17Cl2NO | 322.0760 | 322.0762 | -0.6 | 238 (100), 273 (86), 211 (48) |
| 1b | 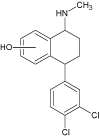 |
5.8 | 322.0760 | 322.0765 | -1.6 | |||
| 2a | 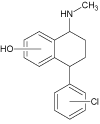 |
4.9 | C17H18ClNO | 288.1150 | 288.1136 | 4.9 | 111 (100), 101 (96), 211 (93), 129 (73) | |
| 2b | 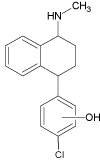 |
3.3 | 288.1150 | 288.1146 | 1.4 | 204 (100), 239 (47) | ||
| 2c | 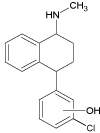 |
3.3 | ||||||
| 3 | 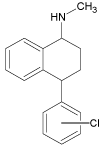 |
6.0 | C17H18ClN | 272.1201 | 272.1193 | 2.9 | 125 (100), 241 (68), 129 (45) | |
| 4 | 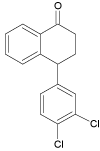 |
10.3 | C16H12Cl2O | 291.0338 | 291.0325 | 4.5 | 117 (100), 145 (50), 238 (45) | |
| 5 | 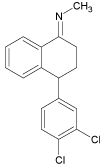 |
5.8 | C17H15Cl2N | 304.0654 | 304.0664 | -3.3 | 129 (100), 287 (86), 263 (48) | |
| 6 | 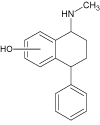 |
3.7 | C17H19NO | 254.1539 | 254.1528 | 4.3 | 239 (100), 101 (84), 133 (37), 223 (29) | |
| 7 | 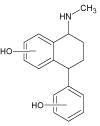 |
4.6 | C17H19NO2 | 270.1489 | 270.1478 | 4.1 | 111 (100), 129 (58), 67 (32), 211 (17), 183 (11), 239 (7) | |
Table 3: Degradation products of SER identified during the irradiation of river water samples by Xe-lamp light.
Continued irradiation of the sertraline solution revealed after 20 minutes formation of three new degradation products (SER 2a–c, SER 3 and SER 4). The compounds SER 2 a-c (m/z 288.1145) were suspected to be formed as a result of the mono-dechlorination of the SER 1a-b molecules. These compounds appear in two peaks indicating the formation of all these three compounds after the same time of irradiation. Furthermore, different fragmentation patterns were observed for products SER 2b,c (tr=3.3 min) and SER 2a (tr=4.9 min) (Figure 6a,b) allowing proper structure elucidation of these analytes. Although SER 2b,c was observed by Pliego et al. [28] during photo- Fenton oxidation, further transformation of SER 2a-c was not observed. The exact structure of these products (specific hydroxylation of aryl ring) could not be defined on the basis of fragmentation data.
The formation of the SER 3 molecule with the m/z 272.1205 suggests that it must be the product of mono-dechlorination of the parent sertraline molecule. Simultaneously the creation of SER 4 (m/z value 291.0340) was observed. The structure of this compound may correspond to the oxidation of the parent molecule of sertraline to the ketone, followed by the elimination of CH3NH2 fragment. This degradation product (SER 4) has already been known in the literature as the product formed after photodegrative destruction as reported by Shen et al. [29].
Further exposition of the sertraline solution to Xe-lamp light caused formation of another degradation product (SER 5, m/z 304.0658) after 40 minutes. This product may correspond to dehydrogenated derivative of the parent molecule. SER 6 (254.1544), appeared after 50 minutes of the irradiation and may corresponds to the full dechlorination of SER 1a-b photoproduct. Shen et al. suggested [29] transformation pathway of sertraline via dehydrogenation to SER 5 followed by hydrolysis to SER 4. However, our study showed that the simultaneous exposition to Xe-lamp light lead to the formation of sertraline ketone (SER 4) after 20 min of irradiation, and compounds SER 5 appears after 40 min. These results prove that this transformation is not possible when using Xelamp, and these photoproducts are formed via different mechanisms.
The final compound detected in this solution (SER 7, m/z value 270.1491) was probably formed by hydroxylation of the SER 6 photoproduct, which was formed from compound SER 1a,b. This transformation chain was not reported in the literature before.
Irradiation of sertraline solution by the xenon lamp has been terminated after 240 minutes. The signal originated from sertraline, SER 1a-b, SER 2b-c, SER 3 and SER 7 has been still recorded, whereas the presence of degradation products SER 2a, SER 4, SER 5 and SER 6 was not detected in the examined solution.
Mass spectra obtained during UHPLC-QTOF-MS analysis for sertraline and photoproducts SER 3 – SER 7 together with proposed structures of fragment ions are provided in the electronic supplementary information (Figures S9−S15).
Dynamics of the transformation products generated during photolysis: The continuous irradiation of a sample containing diazepam let to the formation of nine main photoproducts. As presented in Figure 7a, the first two compounds, DIZ 1 and DIZ 2, are created after 20 min of irradiation in large amount (80% and 20%, respectively, of total relative amount of all 9 photoproducts). Their concentrations slowly decrease proportionally to the irradiation time. DIZ 2 is transformed to DIZ 5, which is degraded after 8 h of exposition, while DIZ 1 is decomposed to unknown compounds. DIZ 3, DIZ 5, DIZ 8 and DIZ 9 are unstable photoproducts formed at low concentration levels, which after continuous irradiation are degraded to unidentified compounds. DIZ 6 and DIZ 7 are two isomers which are formed slowly from 4 h of exposition to Xe-lamp light. Even after the experiment was terminated (after 18 h) they are still present in the sample and the increase of their concentration is noticeable. Although, it was established that DIZ 7 is transformed to DIZ 9, it can be easily observed that product DIZ 7 is more stable and dominant compound.The obtained results are not fully consistent with the results obtained by West et al. [19]. As far as DIZ 2 converts to DIZ 5 during the exposure to Xe-lamp light, the other compounds are formed in different ways form the parent molecule of diazepam, not necessarily from its metabolites. However, the possibility of transformation of diazepam to nordiazepam, known as human metabolite of DIZ, was observed and described here for the first time.
Photolysis of sertraline generated tenanalytes including three isomers of SER 1 and SER 2, however, SER 2a, SER 4 and SER 6 were considered as unstable products since they were created in small amounts and were decomposed while continuing the exposure to Xe-lamp light (Figure 7b). It was established that product SER 6 is transformed to SER 7 what is observed in a decrease of the amount of compound SER 6 whilst SER 7 concentration is increasing. Compound SER 5 is created spontaneously after 40 min of irradiation, however, it is slowly degrading during the continuation of the experiment. One of the isomers, SER 1a, and products SER 3 are formed in the highest amounts and are present in the solution even after the experiment was terminated together with SER 1b, SER 2b,c and SER 7. As it was established product SER 1 is transformed into SER 6 and later to SER 7, which is confirmed by the decrease of the amount of one compound whilst the other concentration is increasing. This transformation pathway was reported here for the first time.
The experiment proved the formation of several degradation products of diazepam and sertraline, some of them appeared persistent and certain transformations are reported here for the first time. It leads to the conclusion that tracing the occurrence of parent and transformed compounds in the aqueous environment as well as in WWTPs is of a great importance and necessary to perform.
Occurrence of photoproducts in environmental waters: The presence of DIZ, SER and their photoproducts was investigated in various water samples: influent and effluent wastewater, treated and untreated water, and river water from Pomerania area (Northern Poland) collected in 5 different months (from February to June). Samples were subjected to SPE procedure described in section ‘Sample collection and preparation’ and the recoveries of the extraction for parent compounds are presented in supplementary materials in Table S1. In order to estimate the actual contamination of the aqueous environment samples were analyzed by UHPLC-QTOF-MS in TargetMS/MS mode. Several parameters were used for proper identification of target compounds, such as accurate mass measurements of (pseudo)molecular ions (< 5 ppm) and at least two fragment ions (< 10 ppm), relative intensity of fragment ions (deviation < 5 %), and compliance of retention times(deviation < 2%).
The obtained results showed that neither parent compounds (DIZ and SER) nor their photoproducts were detected in all analyzed samples. The main reason of non-detection of sertraline and its photoproducts is probability of adsorptions to organic matter during wastewater treatment while applying activated sludge processes [18,30,31]. Diazepam and its transformed compounds are probably removed from the water samples via sorption to sedimenting particles, dilution, advection or rapid photodegradation in superficial water layer [32,33]. It is possible that in natural water samples, in the presence of microorganism and dissolved organic matter, photodegradation processes are more rapid than in laboratory scale leading to total decomposition of parent and transformed compounds causing their non-detection. However, it is also probable that these compounds may infiltrate from stored sludge to groundwater resulting in its contamination. In order to determine the actual pollution of the environment it would be valuable to determine the parent compound together with its degradation products in the sewage sludge, where such pharmaceuticals can be adsorbed. Therefore, further analysis should include determination of both parent and transformed compounds in various matrices, liquid and solid, in order to track the fate of pharmaceuticals in different parts of the aquatic environment.
In this work, a photodegradation of two psychiatric drugs, diazepam and sertraline was investigated with the application of hyphenated technique, UHPLC-QTOF-MS, which provided highly accurate and certain results. The degradation kinetic was investigated for two types of conditions, (1) while exposing to xenon lamp light, and (2) while exposing to natural sunlight in order to simulate process occurring in the environment. The degradation kinetic was pseudo-first order in all cases, however, autocatalytic reactions were observed in case of the second experiment for various natural water samples.
The degradation pathways of DIZ and SER induced by xenon lamp irradiation were proposed, whereas, particular photoproducts and transformations were described here for the first time. Nine photoproducts of DIZ and seven transformed compounds of SER were identified in total, and their presence was investigated in various water samples. However, none of the compounds were detected, probably due to the adsorption of these pharmaceuticals on sewage sludge, sediment or other components of the environment. According to the authors knowledge this is the most accurate and extensive research on photodegradation of diazepam and sertraline with simulation of natural environmental conditions.
This scientific work (as a research project no. 2012/05/N/ST4/01991) has been financially supported within the framework of a grant awarded by the Polish National Science Centre (in the years 2013-2014). Anna Jakimska has been financially supported for the preparation of a doctoral dissertation within the funding of a doctoral scholarship awarded by Polish National Science Centre on the basis of the decisionnumber2013/08/T/ST4/00637. The authors also acknowledge Professor Adriana Zaleska from Department of Chemical Technology (Faculty of Chemistry, Gda?sk University of Technology) for providing access to xenon lamp. Additionally, the authors acknowledge Mr. Andrzej Szwacki for providing water and wastewater samples for this research.
This scientific work (as a research project no. 2012/05/N/ST4/01991) has been financially supported within the framework of a grant awarded by the Polish National Science Centre (in the years 2013-2014). Anna Jakimska has been financially supported for the preparation of a doctoral dissertation within the funding of a doctoral scholarship awarded by Polish National Science Centre on the basis of the decisionnumber2013/08/T/ST4/00637. The authors also acknowledge Professor Adriana Zaleska from Department of Chemical Technology (Faculty of Chemistry, Gda?sk University of Technology) for providing access to xenon lamp. Additionally, the authors acknowledge Mr. Andrzej Szwacki for providing water and wastewater samples for this research.