Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 3
Prevalence of diabetes mellitus is on steady rise, though several therapeutic options are available to treat diabetes mellitus. Medicinal plants are in use alongside prescribed therapies; however more research on medicinal plants is warranted to retrieve their anti-diabetic mechanisms to develop low cost, safe and effective anti-diabetic drugs. Aim of current study is to (a) determine antidiabetic and hypolipidemic potential of organic extract of Musa paradisiaca L. (b) to retrieve underlying mechanisms responsible for anti-diabetic effects triggered by the plant. Alloxan was used to induce diabetes mellitus in rates. Two standard drugs (Glibenclamide 2.5 mg/kg, insulin 4 U/200 mg/dl blood glucose) and various doses of organic plant extract from different morphological parts (100 mg/kg, 250 mg/kg and 500 mg/kg) were administered orally to the groups of healthy and diabetic rats for 20 days. ANOVA followed by t-test was used for the statistical analysis. Organic extract from Musa paradisiaca L. flowers and tracheal fluid have significant hypoglycemic activity. Significantly reduce the levels of bad cholesterol (LDL) while significantly enhance healthy cholesterol (HDL) levels in diabetes rats. Moreover, Organic extract from Musa paradisiaca L. flowers and tracheal fluid may restore normal morphology in the diabetic rats. It is concluded that organic extract of the plant trigger hypoglycemic and hypolipidemic activities possibly through restoring normal morphology and the pancreatic performance in diabetic rats.
M. paradisiaca L.; Diabetes; Histopathology; Hypolipidemic; Anti diabetic
Diabetes mellitus (DM) is a common metabolic disorder, affecting approximately 10% of adult population of the world [1]. Genetic predisposition for compromised insulin action, pancreatic disease, life style, age, ethnicity, surgery, infections, chemicals exposure and environmental factors may play significant role in the development of DM. The burden of the disease is immense owing to DM related blindness, cardiovascular (CVD), kidney, amputation and nerve disease [2]. Adverse effects and high cost of available anti-diabetic therapies warrant the need for new low cost, safe and more affective therapeutic agents [3].
Oxidative stress, triggered by elevated free radicals and reactive oxygen species, plays key role in the development of DM. Medicinal plants containing antioxidants, flavonoids, alkaloids, carotenoids, vitamins, glycosides and terpenoids can both reduce oxidative stress and regulate blood glucose level [4]. Medicinal plants also contain natural α-amylase and α-glucosidase inhibitors which participate both in glucose absorption and digestion to regulate blood glucose level that present an attractive approach to control post-prandial hyperglycemia [5,6]. In low-income and middle-income countries majority of DM patients are still using herbal therapies due to their low cost, less side effects and effectiveness [7].
Studies carried to determine the effectiveness medicinal plants suggest that treatment with herbal remedies may regulate blood glucose levels by impeding carbohydrates abortion, enhancing their metabolism and by improving the performance of pancreatic tissues [3].
Musa paradisiaca L., rich in therapeutically active constituents, is traditionally used for the treatment of diabetes mellitus [8]. Aim of the current study is a) to determine the impact of plant extract on pancreatic morphology, blood glucose & lipids levels and b) retrieve possible underlying mechanism triggered by the plant extract to produce its therapeutic effects.
Collection of plant material
Bract, flower, trachea (flowering stalk) and tracheal fluid of Musa paradisiaca L. were collected during November 2015 from vicinity of District Muzaffargarh Pakistan. Identification and authentication was carried by botanist Dr. Mansoor Hameed Associate Professor Taxonomic Laboratory, Department of Botany, University of Agriculture Faisalabad, Pakistan with specimen number 131-2015. Voucher specimen of each plant part was deposited in the herbarium of Department of Pharmacognosy, Faculty of Pharmacy, and University of Karachi for further reference.
Extraction of plant material
The collected parts (bract 250 g, flower 250 g and flowering stalk 200 g) of the plant were washed with clean water and dried in shade at room temperature. The dried parts were powdered coarsely in a mechanical grinder separately and macerated in a methanol for 7 days in glass bottles with intermittent shaking. After filtration liquid filtrate was concentrated and evaporated to dryness at 40°C by using a rotary evaporator (Rotavapor R-200, Buchi). The milky fluid (700 mL) collected in ambered coloured bottles from floral stalk (tracheal fluid) by cutting the bunch of fruit is lyophilized at -65 to -60°C with vacuum of 30-40 milibar in alpha 1-4LSC Christ Germany lyophilizer. The dried materials were weighed (bract 25 g, flower 27 g, flowering stalk 20 g, tracheal fluid 17 g) labeled and stored in biomedical freezer (Sanyo biomedical freezer, MDF-U333, Japan).
Animals Preparation
Experiments were performed on 96 healthy adult ’ s albino Wistar rats; age 2-3 months and body weight 150-200 g. The animals were housed individually in polypropylene cages in animal house facility of Faculty of Pharmaceutical sciences, Government College University Faisalabad at temperature (21-23°C) under a constant 12 h light and dark cycle and relative humidity (35-60%) with free access to standard balanced commercial pellet food and water ad libitium. These animals were allowed one week to acclimate to the facilities prior to use in any experiments. Experimental protocols were approved by Institutional Animal Ethics Committee of Government College University Faisalabad vide letter No Pharm/15/1928 were in strict accordance with institutional animal ethical committee guidelines for the care and use of laboratory animals.
Chemicals and Equipment
Insulin (Mixtard 30, Novo Nordisk), Alloxan mono hydrate (Aldrich), Glibenclamide (Pharmedic laboratories (Pvt) Lahore), Glucometer (Accu-Chek active) Roche Mannhein Germany. Commercial kit Trig, Commercial kit CHOL2, Commercial kit HDLC3 (Roche Pakistan Limited). All other chemicals used in this experiment were obtained from Sigma Chemical Company (St. Louis MO, USA) was of analytical grade.
Induction of diabetes mellitus
Diabetes was induced by the method with slight modification [9,10]. Adult albino Wistar rats were fasted for 24 hours then diabetes was induced by a single intraperitoneal injection of a freshly prepared alloxan monohydrate solution (140 mg/kg) in ice cold 0.9% NaCl solution. The animals were fed with 2 mL 5% dextrose solution using orogastric tube to overcome the drug induced hypoglycemia immediately. Blood glucose levels were measured after 72 hours of the injection for the confirmation of hyperglycemia. Animals with blood glucose level more than 150 mg/dl were categorized as diabetic. Glibenclamide (2.5 mg/kg) was used as the standard drug [10,11]. Insulin dose was adjusted according to Sena et al. [12].
Experimental procedure
Anti-diabetic effects induced by methanolic extracts of different morphological parts; bract, flower, trachea and tracheal fluid of Musa paradisiaca L. were measured in accordance with Jain’s method [9] (Jain et al., 2010) with slight modification. Ninety diabetic and six healthy Wistar albino rats were weighed and divided into 16 groups having six animals in each group. Group 1 was administered 0.9% (w/v) NaCl orally and marked as healthy control group. Group 2 was diabetic control group, receiving orally (0.9% NaCl w/v); Group 3: diabetic rats administered glibenclamide (2.5 mg/kg) daily for 20 days orally; Group IV: Diabetic rats were administered with subcutaneous injection of insulin at the dose of 4 U/200 mg/dl sugar level. Group 5 to 16 of diabetic rats were orally administered orally with 100, 250 and 500 mg/kg extract of bract, flower, trachea and tracheal fluid of Musa paradisiaca L. respectively daily for twenty days. Fasting blood glucose and weight of each animal was estimated on days 0, 5, 10, 15 and 20 of treatment. The other biochemical parameters were determined on day 20 after the animals were sacrificed [9].
Blood and tissue sample collection
On the final day of study (20th day) rats in each group were housed in metabolic cages having free access to water and food and were fasted overnight. These animals were sacrificed under chloroform anaesthesia. Blood samples were collected and centrifuged at 3000 r/min for 10 min to obtain the serum. The serum was stored below 4°C until further use for biochemical determinations. Pancreas were surgically excised and fixed in 10% buffered formalin for histological analysis [12].
Biochemical analysis
Blood glucose, Triglycerides (TGs), Total Cholesterol (TC), High Density Lipoprotein Cholesterol (HDL-C) concentrations in serum was determined by using commercial kits. Low Density Lipoprotein Cholesterol (LDL-C) and Very Low Density Lipoprotein (VLDL-C) was calculated by the Friedewalds formula as described below.
VLDL=TG/5.
LDL=TC-(HDL+VLDL)
Histo-pathological investigations
Pancreases were fixed in 10% neutral buffered formalin for a period of 24 hour immediately after dissection and then dehydrated in graded alcohol (30%-100%). Afterwards embedded in paraffin and cut into slices of 2 × 2 mm to 1 × 2 cm and thickness of 3 mm with the help of Sakura Accu-cut: SRM 200 CW and stained with Mayer hematoxylin and eosin (H and E) stain. Slides were named and examined under microscope for the morphological changes in pancreas [14].
Statistical analysis
All results are expressed as mean ± SD. To determine the effect of treatment of different doses of extracts data was analyzed by using one-way analysis of variance (ANOVA) followed by turkey test. P-Value>0.05 was considered statistically significant.
Methanolic extract from different morphological parts i.e., bract, flower, trachea and tracheal fluid at the dose of 100, 250, 500 mg/kg were given to the diabetic rats for 20 days continuously. Weight and blood fasting glucose levels were measure at 0, 5, 10, 15 and 20th day. Biochemical parameters such as (total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, VLDL, TC/HDL, LDL/HDL) and histological study of pancreas were performed to evaluate the biochemical and morphological effects triggered by organic extracts of the plant.
Blood glucose lowering activity from the extracts of Musa paradisiaca L. bract, flower, trachea and tracheal fluid were compared with standards drugs (Insulin and Glibenclamide). Tracheal fluid and flower extract showed significant blood glucose lowering activity as shown in (Figure 1 and Figure 2) whereas trachea have low blood glucose regulating activity (Figure 3) and methanolic extract of bract have no effect on blood glucose level (Figure 4).
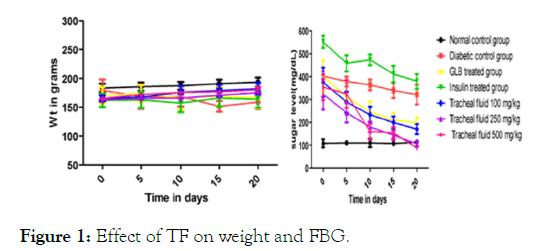
Figure 1: Effect of TF on weight and FBG.
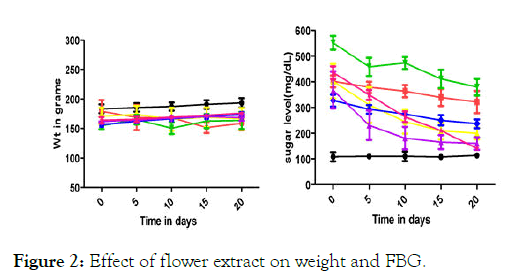
Figure 2: Effect of flower extract on weight and FBG.
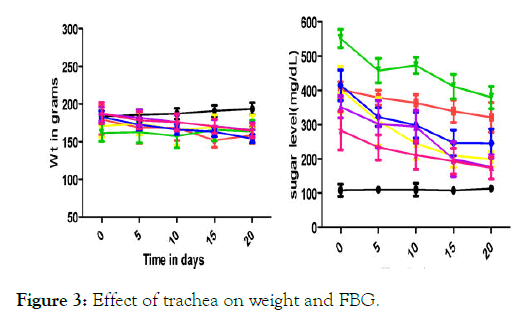
Figure 3: Effect of trachea on weight and FBG.
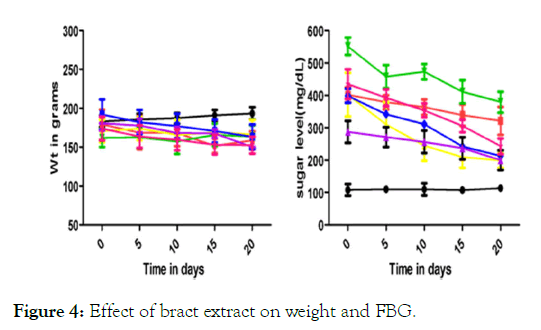
Figure 4: Effect of bract extract on weight and FBG.
Different physical parameters such as water intake, food intake and body weight were also observed. Diabetic rats treated with different doses of tracheal fluid and flower‘s methanolic gain weight (Figures 1 and 2). Whereas weight loss was observed in diabetic rats treated with methanolic extract from bract and trachea (Figure 4 and Figure 3).
We measured the levels of serum cholesterol, triglycerides and LDL to determine the effect of methanolic extract Musa paradisiaca L. tracheal fluid, flowers, bract and trachea on these parameters. Methanolic extract from Musa paradisiaca L. tracheal fluid and flowers significantly decrease the level of serum cholesterol, triglycerides and LDL, while level of HDL were significantly elevated as shown in (Figure 5a-5d and Figure 6) whereas the effect from bract and trachea (flowering stalk) were not significant (Figure 7 and Figure 8).
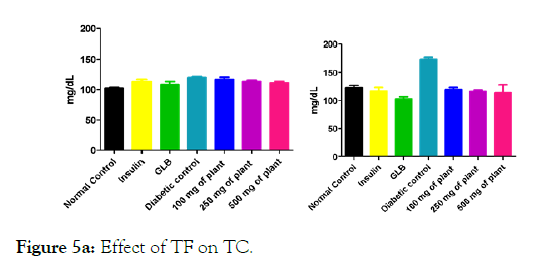
Figure 5a: Effect of TF on TC.
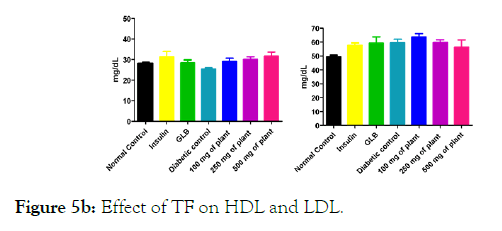
Figure 5b: Effect of TF on HDL and LDL.
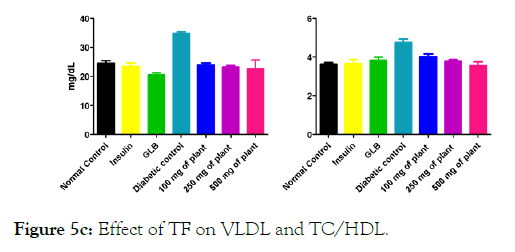
Figure 5c: Effect of TF on VLDL and TC/HDL.
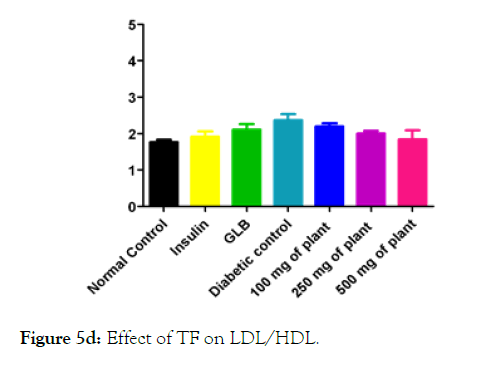
Figure 5d: Effect of TF on LDL/HDL.
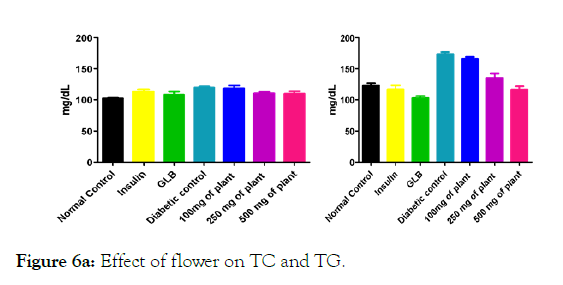
Figure 6a: Effect of flower on TC and TG.
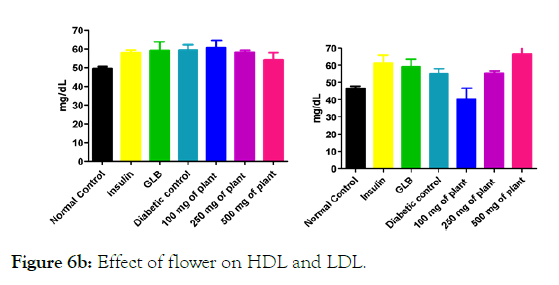
Figure 6b: Effect of flower on HDL and LDL.
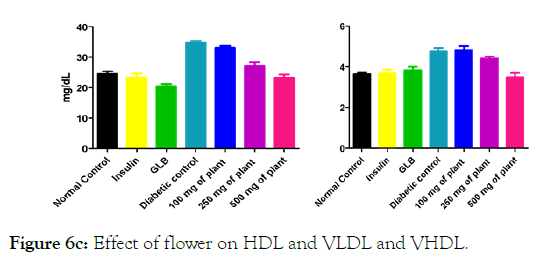
Figure 6c: Effect of flower on HDL and VLDL and VHDL.
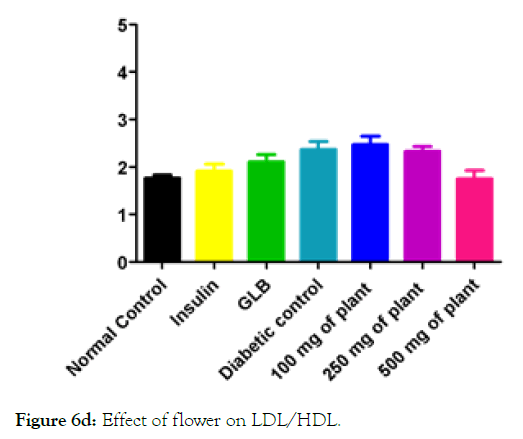
Figure 6d: Effect of flower on LDL/HDL.
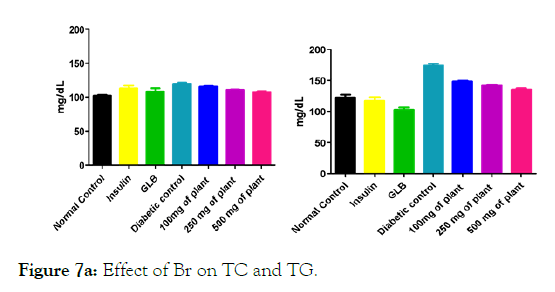
Figure 7a: Effect of Br on TC and TG.
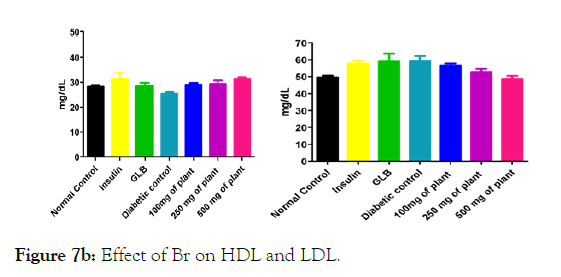
Figure 7b: Effect of Br on HDL and LDL.
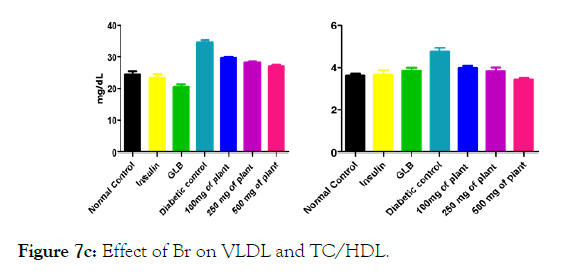
Figure 7c: Effect of Br on VLDL and TC/HDL.
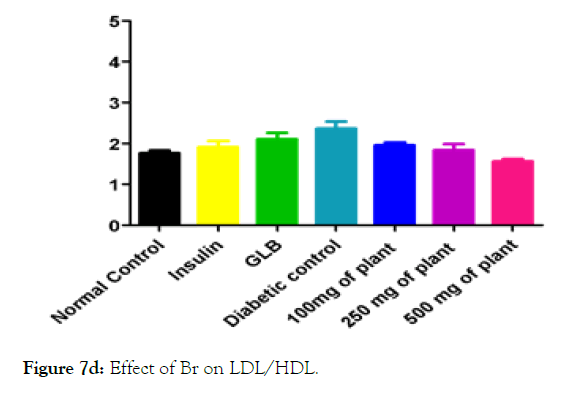
Figure 7d: Effect of Br on LDL/HDL.
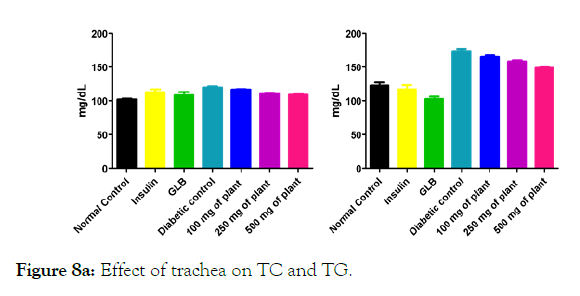
Figure 8a: Effect of trachea on TC and TG.
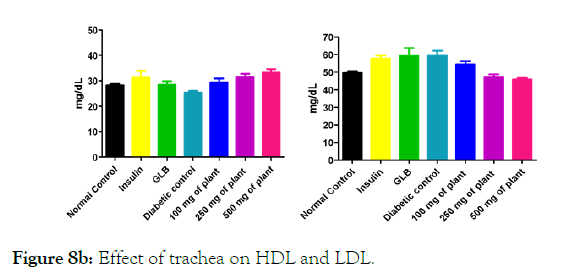
Figure 8b: Effect of trachea on HDL and LDL.
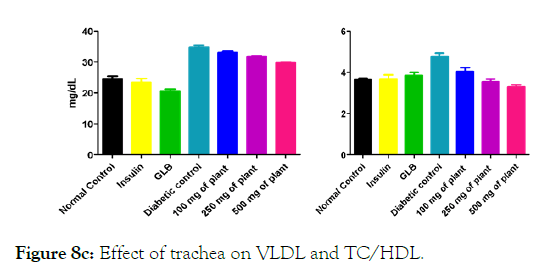
Figure 8c: Effect of trachea on VLDL and TC/HDL.
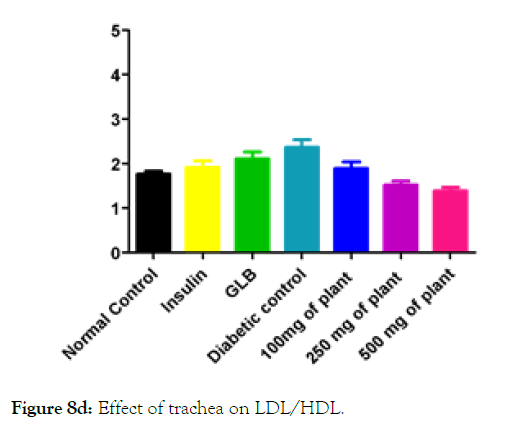
Figure 8d: Effect of trachea on LDL/HDL.
In order to investigate possible underlying mechanisms triggered by Musa paradisiaca L. responsible for its hypolipdimic and antidiabetic activities. We treated diabetic rates with the methanolic extract of Musa paradisiaca L. and analyzed morphology of the pancreas. Histopathological investigation shows that healthy rat’s pancreas shows Pancreatic Ducts and Islets of Langerhans with scattered β cells are normal in shape surrounded by visible red blood cells. Scattered small intra lobular ducts and large intra tubular ducts are surrounded by dense connective tissue are also present marked by ‘a’ as shown in (Figure 9A).
While degenerative changes in variable degree with scent colloid and congestion in the cells, Inflammation and hypertrophy of islet of Langerhans with lymphocytic infiltration in diabetic control group marked by ‘b’ shown in (Figure 9A). Pancreas of insulin treated diabetic rats showed normal islets with intact β cells whereas pancreas of Glibenclamide treated diabetic rats show normal pancreatic granulated islets with non-appearance of dilation marked by ‘d’ and ‘c’ respectively shown in (Figure 9A).
Diabetic rats treated with methanolic flower extract (100 mg/kg) show improved pancreatic folliculated epithelial cells and dilated follicles while at the dose of 250 mg/kg improved large islets with clear beta cell granules are prominent. Diabetic rats treated with higher dose of the extract (500 mg/kg) restore normal pancreatic morphology with prominent lining epithelial cells of acini with thick cells in the lumen of acini marked by ‘h’, ‘i’ and ‘j’ in (Figure 9B).
Diabetic rats treated with 100 mg/kg of tracheal fluid showed improvement in pancreatic morphology; islet cells are prominent with congestion in blood vessels whereas treatment with 250 mg/kg shows evenly distributed β cells with increase in number, normal acinoid and islets of langerhan. Diabetic rats treated with 500 mg/kg show restored normal pancreatic morphology; compact islets of Langerhans with prominent nuclei surrounded by acinar with well-arranged lobules that surround islets cells are marked with n, o and p are shown in (Figure 9D).
Histopathology of diabetic rats treated with different concentration methanolic extract of bract and trachea show no or little effects on pancreatic morphology; treatment with 100 mg/kg show as moderate to severe atrophy of eosinophil, necrotic changes in acini and showing shrinkage in acini whereas 250 mg/kg extract shows moderate necrotic changes with prominent vascularity of islet cells and pancreas of rats that were treated with 500 mg/kg shows atrophy and collapse of follicles marked with e, f and g in the (Figure 9A) and k, l and m in (Figure 9C-9F) respectively. It suggests that methanolic flower’s extract and tracheal fluid can rectify diabetes induced anomalies and restore normal morphology of pancreas while treatment with methanolic extract of bract and trachea show no or little effects on pancreatic morphology.
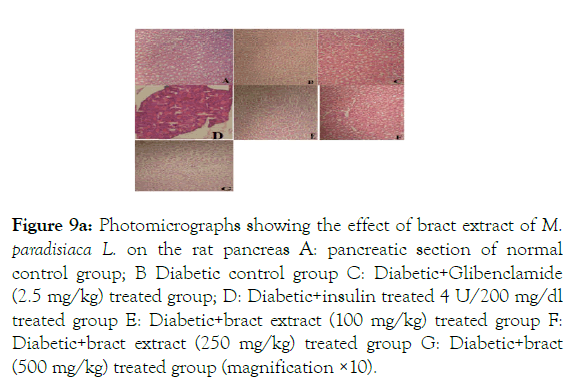
Figure 9a: Photomicrographs showing the effect of bract extract of M. paradisiaca L. on the rat pancreas A: pancreatic section of normal control group; B Diabetic control group C: Diabetic+Glibenclamide (2.5 mg/kg) treated group; D: Diabetic+insulin treated 4 U/200 mg/dl treated group E: Diabetic+bract extract (100 mg/kg) treated group F: Diabetic+bract extract (250 mg/kg) treated group G: Diabetic+bract (500 mg/kg) treated group (magnification ×10).
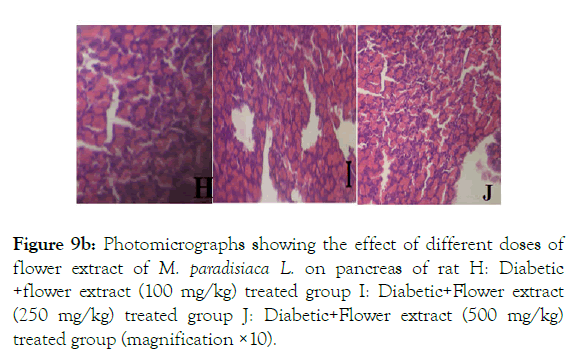
Figure 9b: Photomicrographs showing the effect of different doses of flower extract of M. paradisiaca L. on pancreas of rat H: Diabetic +flower extract (100 mg/kg) treated group I: Diabetic+Flower extract (250 mg/kg) treated group J: Diabetic+Flower extract (500 mg/kg) treated group (magnification ×10).
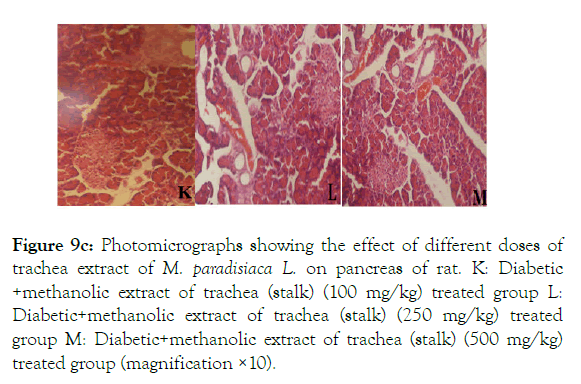
Figure 9c: Photomicrographs showing the effect of different doses of trachea extract of M. paradisiaca L. on pancreas of rat. K: Diabetic +methanolic extract of trachea (stalk) (100 mg/kg) treated group L: Diabetic+methanolic extract of trachea (stalk) (250 mg/kg) treated group M: Diabetic+methanolic extract of trachea (stalk) (500 mg/kg) treated group (magnification ×10).
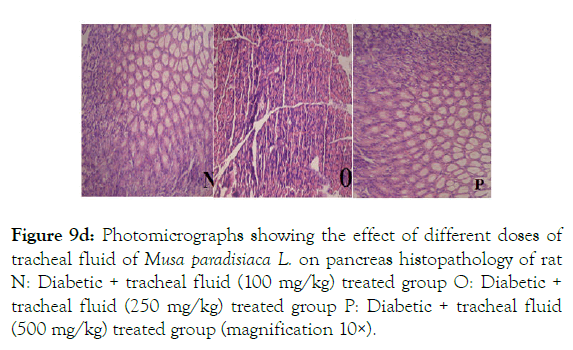
Figure 9d: Photomicrographs showing the effect of different doses of tracheal fluid of Musa paradisiaca L. on pancreas histopathology of rat N: Diabetic + tracheal fluid (100 mg/kg) treated group O: Diabetic + tracheal fluid (250 mg/kg) treated group P: Diabetic + tracheal fluid (500 mg/kg) treated group (magnification 10×).
Medicinal plant and their remedies are used for the treatment of diabetes in traditional system due to their low cost, lower side effects and effectiveness. Our results support the notion that medicinal plants are effective against elevated blood glucose and lipid levels. Musa paradisiaca L. flower ’ s extract and tracheal fluid significantly reduce blood glucose level and restore normal pancreatic morphology while treatment with bract and trachea extract show no or little effects on both blood glucose levels and pancreatic morphology. It suggests that Musa paradisiaca L. flower and tracheal fluid may significantly reduce blood glucose levels by restoring normal pancreatic morphology in diabetic rats. Morphological recovery of pancreas may restore its performace that may presents one possible underlying mechanism for anti-diabetic effects triggered by flower’s extract and tracheal fluid from Musa paradisiaca L.
Generally body weight decrease in diabetic patients but increase in body weight and decrease in the blood glucose level is due to the secretion of insulin from remnant pancreatic cells and improve in glycemic control mechanism [15].
Musa paradisiaca L. flower and tracheal fluid also significantly increase good cholesterol (HDL) and significantly decrease bad (LDL) cholesterol levels to reduce diabetes associated risk for heart diseases. The Diabetic rats received tracheal fluid and flower methanolic extract gain weight. While weight loss was observed in diabetic rats received methanolic extract from both trachea and bract.
Plants show their anti-diabetic action by restoring the pancreatic tissue function or/ and inhibit glucose absorption from intestine and/or by increasing insulin secretion [9]. Findings from this study suggest that Musa paradisiaca L. flower and tracheal fluid have glucose lowering effect and regulate pancreatic biochemical and patho-physiological parameters associated with diabetes. These extract may ensure pancreas protection to boost its performance to regulate elevated blood glucose level in diabetes animals. It suggest that possible cellular mechanisms triggered by plant extracts leads to improved glycogenesis or/and enhanced insulin secretion and consequently regulate blood glucose levels.
Alloxan model was used due to its lower cost, high reproducibility, fast decrease in liver glycogen level and reversible effects within three months.
In the present study two drugs Glibenclamide and insulin were used as standards. Glibenclamide decreases blood glucose by increasing the secretion of insulin from the pancreas. The medicine is only effective when some β-cells are damage but it has no effect at all when all the β-cells are destroyed. Musa paradisiaca L. flower and tracheal fluid may act through this possible mechanism as may be few β cells are still surviving [16]. Hypoglycemic effect of the flower and tracheal fluid can be triggered by several mechanisms such as increase uptake and oxidation of glucose in peripheral tissues, inhibition of α- glucosidase, increase in insulin sensitivity and control of lipid metabolism.
Musa paradisiaca L. flower and tracheal fluid prevent the dyslipidemia with reference to standard drug. Ethno medicines have long history to be used as blood glucose decreasing abnormalities, but no work is carried out to retrieve underlying mechanisms. Current study not only document plant triggered hypolipdimic and antidiabetic activities but also presents possible underlying mechanism for these effects. In this study we documented the effect of organic extract from Musa paradisiaca L. flowers and tracheal fluid have significant hypolipdimic and antidiabetic activities while organic extract from Musa paradisiaca L flowers and tracheal fluid can restore normal morphology in the pancreas of diabetic rats suggesting that morphological recovery of diabetic pancreas present one possible underlying mechanism for anti-diabetic effects triggered by methanolic extract from Musa paradisiaca L.
We concluded that methanolic extract from Musa paradisiaca L. are significantly effective against elevated blood glucose and lipid levels. Moreover, tracheal fluid and methanolic extract from Musa paradisiaca L. flower may trigger their glucose lowering effect by restoring normal morphology in the pancreas of diabetic rats. These extracts significantly increase HDL and significantly decrease LDL that may highlight their ability to render diabetes associated heart diseases. These findings suggest that flower and tracheal fluid of Musa paradisiaca L. is excellent candidate for the further study on diabetes mellitus. These parts may be used for the development of low cost, safe and effective pharmaceutical preparation for diabetic patients.
The authors are very thankful to Dean Faculty of Pharmacy and Pharmaceutical sciences, University of Karachi for providing the facilities to carry out the research work.
The authors have no conflict of interest.
Citation: Khizar A, Rizwani GH, Hina Z, Shareef H, Taqi MM (2019) Musa paradisiaca L. May Restore Pancreatic Morphology and Function to Trigger its AntiDiabetic and Hypolipidemic Activities in Alloxon-Induce Diabetic Rats. Med Aromat Plants (Los Angel) 8: 333. doi: 10.35248/2167-0412.19.8.333
Received: 13-Jun-2019 Accepted: 21-Jun-2019 Published: 27-Jun-2019 , DOI: 10.35248/2167-0412.19.8.333
Copyright: © 2019 Khizar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.