Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2020)Volume 8, Issue 1
Introduction: This study was conducted to evaluate the effectiveness of the mycobiome diet (as presented in the book Total Gut Balance) on the human gut microbiome in general and the gut mycobiome (fungal community) in particular. Enrolled subjects were evaluated for improved health, gastrointestinal symptoms, and weight loss, as well as subjective reports of changes in energy, fatigue, and sleep.
Method: Ten healthy volunteers (six males and four females ranging in age from 30 to 70) were enrolled in this 28-day protocol. Participants completed a food journal, checking off daily and weekly required foods, as well as noting bowel movements, weight, and any digestive-related complications. Fecal samples were collected at the beginning and end of the study, with mycobiome and bacteriome profiles sequenced using ITS and 16S regions, respectively.
Results: The mycobiome diet was highly successful at reducing pathogenic Candida species. Within two weeks, Candida species overall decreased by 72.4%; C. albicans in particular decreased 1.42-fold, while C. tropicalis was undetected after 4 weeks. Subjects significantly increased their levels of beneficial bacteria, specifically Faecalibacterium prausnitzii, Bifidobacterium, Roseburia, Lactobacillus, and Bacteroides. Furthermore, pathogenic bacteria decreased significantly, including Escherichia coli, Bacteroides fragilis, and Clostridium. The changes in the microbiome structure were accompanied with improvement in digestive symptoms, weight loss, less fatigue, more energy, better sleep, and fewer cravings for empty-calorie foods.
Conclusion: Our data showed that adhering to the mycobiome diet for 4 weeks led to positive shifts in fungal and bacterial microbiome communities concurrently with positive improvement in GI symptoms and overall health.
Mycobiome diet; Gut health; Candida species
Recent advances in DNA sequencing technology have allowed characterization of the human microbiome, a collection of microbial communities composed of bacteria, fungi, and viruses. Studies have demonstrated that each person has a unique microbial gut profile that is influenced by lifestyle, as well as various innate and environmental factors. Specifically, diet has been shown to play a central role in influencing the microbial composition of the gut, altering the balance between beneficial and harmful levels of microorganisms. The typical western diet includes a high percentage of fats, simple/refined sugars, salt, and animal protein, with a low percentage of fiber [1]. This eating pattern can lead to dysbiosis or imbalance in the microbiota, resulting in obesity and may increase the risk of development of such wide-ranging conditions as diabetes, arthritis, inflammatory bowel disease (IBD), heart disease, and even cancer [2].
Recent studies have shown that rebalancing and maintaining the bacterial and fungal communities of the gut microbiota is a prerequisite for optimal gastrointestinal health and overall wellness. To achieve this aim, we designed the mycobiome diet (see Total Gut Balance) with the purpose of having a total gut balance of bacterial and fungal communities [3-6]. The mycobiome diet takes the best elements from the Paleo, low-carb, vegetarian, and mediterranean diets, while avoiding aspects of each of these diets that have specifically been proven to increase pathogenic fungi in the human gut. It avoids the vegetarian diet’s often high consumption of sugar, which could lead to increased growth of Candida in the gut [7], while fully utilizing the prebiotic fibers in vegetables that benefit the balance of beneficial bacteria. Further, the mycobiome diet avoids the low carbohydrate diet’s typical heavy reliance on red meats, which have been implicated as a factor in developing several chronic illnesses including colorectal cancer [8], while taking advantage of the many fungal-friendly aspects of seafood. Also, it borrows from the Paleo-style diet’s whole natural food philosophy, but does not avoid fiber-rich and nutrient-dense legumes, which provide an excellent source of food (specifically fiber and resistant starch) for the beneficial members of the microbiome. The mycobiome diet furthermore avoids the mediterranean diet’s overuse of complex carbohydrates and grains, which increase the prevalence and abundance of harmful fungi, as well as its penchant for alcohol, which has been proven to decrease microbial diversity [9].
This study was conducted to evaluate the effectiveness of the mycobiome diet on the human gut microbiome in general and the gut mycobiome (fungal community) in particular. In addition, the study captured the effect of the diet on gastrointestinal symptoms and subjective markers including weight loss, fatigue, energy, sleep, and cravings for empty-calorie foods.
Ten healthy volunteers (six males and four females ranging in age from 30 to 70) completed informed consent and were enrolled in the study. Participants completed a food journal, checking off daily and weekly required foods, as well as noting bowel movements, weight, and any digestive related complications. General rules for the study were as follows:
1. Consume mostly whole food. No processed foods or packaged foods with more than three ingredients.
2. Include a “good” protein with every meal and snack (lean or plant-based, no processed or cured meat.)
3. Include a “ good ” fat source with every meal and snack (approved oils or fat-rich foods primarily containing monounsaturated and polyunsaturated fats, with low saturated fat content).
4. Include a fiber-rich and/or resistant-starch-rich food (whole grains, legumes, and starchy vegetables) at each meal, but do not have more than one serving of these foods at any one meal. Spread these foods throughout the day in smaller servings, to avoid excess carbohydrates at any given meal that could potentially increase the abundance of Candida species.
5. Do not change exercise, sleep, supplement regimen, or stress management habits during the course of this study.
Fecal samples were collected at the beginning and end of the study, with mycobiome and bacteriome profiles sequenced using ITS and 16S regions, respectively. Bacterial and fungal DNA was isolated and purified using QiaAmpFast DNA Extraction Kit (Qiagen). The quality and purity of the isolated genomic DNA was confirmed using the NanoDrop 2000 device (Fisher Scientific). DNA concentration was quantified using the Qubit 2.0 instrument applying the Qubit dsDNA HS Assay (Life Technologies) and normalized to 100 ng per sample. Following denaturation, the amplicon library was generated with sheared PCR products using Ion plus Fragment Library kits (<350 bp) and barcoded with Ion Xpress™ Barcode Adapter, ligated with the A and P1 adaptors. Barcoded libraries were concentrated, pooled, and sequenced on the Ion Torrent PGM system using the Ion Sequencing 400 bp Hi-Q View Kit (Life Technologies, USA). Classification at the species level was referenced using the Greengene (ver. 13.8) reference database and taxa assigned using the nBlast method with a 90% confidence cut-off.
Adherence to the mycobiome diet led to changes in the mycobiome structure at different taxonomic levels
Figure 1 summarizes the mycobiome profile (fungi in the gut) of subjects before and after following the mycobiome diet. Analysis showed that within two weeks of beginning the mycobiome diet, a decrease of 72.4% in the abundance of members of the Candida genus was noted. Moreover, C. albicans decreased by 142%, while C. tropicalis was no longer detectable from any participant’s samples collected at 4 weeks.
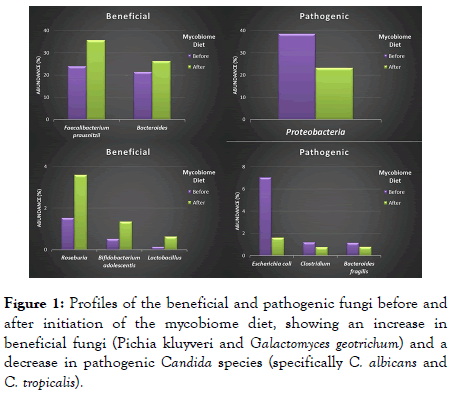
Figure 1. Profiles of the beneficial and pathogenic fungi before and after initiation of the mycobiome diet, showing an increase in beneficial fungi (Pichia kluyveri and Galactomyces geotrichum) and a decrease in pathogenic Candida species (specifically C. albicans and C. tropicalis).
In addition to the decrease in the abundance of pathogenic Candida, we also noted an increase in beneficial fungal species by week 4. For example, Galactomyces geotrichum and Pichia kluyveri increased by 58.4% and 45.1%, respectively.
Adherence to the mycobiome diet led to changes in the bacteriome structure at different taxonomic levels
Figure 2 shows the abundance of the main bacterial phyla detected in the gut microbiota of individuals enrolled in the study before and after following the mycobiome diet. At baseline, the bacterial phyla were composed mainly of Firmicutes, Bacteroidetes, and Proteobacteria. Of note, the level of proteobacteria was elevated in these baseline samples (a marker of potential dysbiosis and inflammation when elevated) [10]. Decreases in relative abundance were observed as early as 2 weeks after the initiation of the mycobiome diet. Analysis of fecal samples after 4 weeks of diet consumption showed a 1.7-fold decrease in the abundance of the pro-inflammatory proteobacteria (levels reduced from 38.6% to 23.3%). This reduction in turn led to a favorable higher relative abundance of the other two bacterial phyla (Firmicutes and Bacteroidetes).
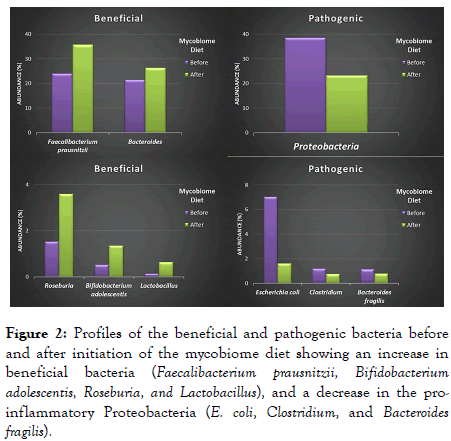
Figure 2. Profiles of the beneficial and pathogenic bacteria before and after initiation of the mycobiome diet showing an increase in beneficial bacteria (Faecalibacterium prausnitzii, Bifidobacterium adolescentis, Roseburia, and Lactobacillus), and a decrease in the proinflammatory Proteobacteria (E. coli, Clostridium, and Bacteroides fragilis).
Like the majority of the population in the U.S., our study subjects came into the trial with high levels of pro-inflammatory Proteobacteria (in a survey of nearly one thousand subjects with no underlying diseases we observed that 63% of these participants had a high level of this phylum (data not shown). Analysis of the bacterial profiles at the genus/species levels showed an increase in bacteria that are considered beneficial, including Faecalibacterium prausnitzii, Bifidobacterium adolescentis, Roseburia, Lactobacillus, and Bacteroides (Figure 2). In this regard, F. prausnitzii and B. adolescentis had an increase of 35.8% and 61.6%, respectively. Additionally, the genera Lactobacillus and Roseburia increased by 77.6% and 57.5%, respectively. In contrast, the abundance of known bacterial pathogens, including Escherichia coli, B. fragilis, and Clostridium, decreased. These populations decreased by 74%, 45.3%, and 55.5% in E. coli, B. fragilis, and Clostridium, respectively, by week 4.
Adherence to the mycobiome diet led to subjective improvements in participants’ gastrointestinal symptoms and other health markers
Before starting the study, 60% of the participants reported health issues, especially gastrointestinal symptoms. Thirty percent had SIBO (small intestinal bacterial overgrowth, which occurs when bacteria that normally grow in other parts of the gut start growing in the small intestine), and 10% had celiac disease. Many reported problems with gas, bloating, constipation, heartburn, and diarrhea. Other reported issues included fatigue, low energy, cravings for sugar, bread, or salty carbs, and sleep disturbances, especially waking in the middle of the night.
At the end of the study, all participants with GI symptoms reported moderate or dramatic improvements. In this regard, all participants who wanted to lose weight did lose weight (between two and 10 pounds). Thirty percent of the participants reported moderate or dramatically improved fatigue and higher energy levels, and 30% reported better sleep, less waking at night, and reduced hot flashes. Furthermore, 60% of the participants decided to continue the mycobiome diet after the trial. Of the 40% who returned to their former diets, all but one subject (whose diet was already quite similar to the mycobiome diet) experienced worsening of their symptoms. After symptom recurrence, some decided to go back on the mycobiome diet again or return to certain aspects of the diet that they believed were helpful.
The mycobiome diet rebalances the fungal community in the gut
Our study showed that adherence to the mycobiome diet led to changes in fungal as well as bacterial communities. The decrease in the abundance of pathogenic fungi (eg., C. albicans and C. tropicalis) is encouraging in that these yeasts are known to be associated with a multitude of diseases such as inflammatory bowel diseases (IBD) and Crohn’s disease [11,12]. Candida over-representation in the gut is accompanied by an increase of inflamed intestinal tissue and may also prevent recovery of beneficial bacteria such as Lactobacillus from a disturbance in the gut bacteriome. Additionally, the presence of C. tropicalis in an IBD setting leads to greater severity of IBD symptoms and production of pro-inflammatory cytokines [13,14].
In addition to a decrease in the levels of pathogens, adherence to the mycobiome diet led to a simultaneous increase in beneficial fungi particularly, Pichia kluyveri and Galactomyces geotrichum. In 2014, our group conducted an analysis of interactions in the microbiome of HIV-infected patients, which lead to the discovery of an antagonistic relationship between Pichia (a nonpathogenic yeast that is used in Europe as a biocontrol agent to protect agricultural crops from fungal growth) and Candida [15]. We noted that a decrease in the level of Pichia coincided with an increase in Candida colonization and vice versa. Upon further testing we found that Pichia released an inhibitory molecule that was capable of limiting the growth of Candida and also other fungal pathogens such as Fusarium and Aspergillus. Moreover, Pichia cells, or the medium in which they had been grown, resulted in significant inhibition of biofilm formation by Candida. Finally, we were able to treat an infection caused by C. albicans using Pichia filtrate. This data suggests that increasing levels of Pichia by adherence to the mycobiome diet will help keep Candida in check and may explain the improvement of symptoms noted in our volunteers.
Galactomyces geotrichum is another beneficial fungus that was increased in participants on the Mycobiome diet. In our study comparing the gut mycobiome of Crohn’s disease (CD) patients with their non-Crohn’s relatives (NCDR), we showed that C. tropicalis was common in the CD group (10%), while Saccharomyces cerevisiae and G. geotrichum (27% and 8%, respectively) were the most abundant in the NCDR group [16]. A recent study showed that G. geotrichum strongly binds with lactic acid bacteria, suggesting a potential role in maintaining the symbiotic microbiota in a healthy gut [17]. Another benefit attributed to G. geotrichum is its ability to produce peptides that could inhibit angiotensin I-converting enzyme which is responsible for blood pressure regulation in humans. Taken together, this suggests that having an increase in the abundance of this fungal species as a result of adhering to the mycobiome diet may help in improving the total microbiome gut balance.
The mycobiome diet rebalances the bacterial community in the gut
Participants in the mycobiome diet trial experienced a shift in the bacteriome, with a decrease in harmful bacteria (Proteobacteria, Escherichia coli, Bacteroides fragilis, and Clostridium) accompanied by an increase in beneficial bacteria (Faecalibacterium prausnitzii, Roseburia, Lactobacillus, Bacteroides, and Bifidobacteria).
A number of studies support the concept that an overabundance of Proteobacteria in the GI tract reflects dysbiosis or an unstable gut microbiome structure. It has been suggested that disruption of homeostasis by host or other environmental factors (eg., a low-fiber diet and acute or chronic inflammation) could cause dysbiosis, and an accompanying increase in the abundance Proteobacteria could lead to inflammatory symptoms or invasion by pathogens. In this regard, IBD dysbiosis is characterized by an increase of Proteobacteria with a corresponding decrease in representation of Firmicutes [18]. Animal studies suggest that the microbial imbalance in IBD is associated with the over-representation of pathogenic Proteobacterial taxa such as Enterobacteriaceae (eg., E. coli) and a reduction in protective Firmicutes species such as F. prausnitzii [19].
Other studies have also demonstrated a decrease in Firmicutes diversity, with fewer constituent species detected in patients with IBD compared with controls [20]. Changes in the two dominant phyla, Firmicutes and Bacteroidetes, are coupled with an increase in abundance of members of the Proteobacteria phylum, which have been increasingly found to have a key role in IBD [21]. Studies have shown a shift towards an increase in species belonging to this phylum, suggesting an aggressor role in the initiation of chronic inflammation in patients with IBD. More specifically, increased numbers of E. coli, including pathogenic variants, have been documented in ileal CD [22].
E. coli is a pathogenic bacterium that has been shown to play an important role in inducing chronic intestinal inflammation in susceptible hosts, where it can differentially induce TH1 and TH17 cell inflammatory responses [23]. Our team also showed that this organism is elevated in Crohn’s disease patients. Additionally, we demonstrated that E. coli cooperates with C. tropicalis and Serratia marcescens to form biofilms in vivo in animals with IBD [24]. Furthermore, E. coli has been shown to work with B. fragilis (another pathogenic bacterium reduced by the Mycobiome diet) to form biofilms in the GI tract that could lead to colon cancer. These studies are beginning to show the potential role of the colon microbiota in cancer development [25].
Studies linking increased abundance of E. coli have shown that higher consumption of snack and junk food products results in an increase of E. coli counts and suppression of beneficial lactobacilli and butyrate-producing Firmicutes members (eg., F. prausnitzii). These changes represent a potential detrimental inflammatory gut microbiota milieu for the host [9]. In contrast, a long-term polysaccharide-rich diet (high in fiber and resistant starch) has been linked to lower abundance of members of the Enterobacteriaceae taxa (Shigella and Escherichia).
Recent studies suggest that certain species of Bacteroides are beneficial, providing breakdown of complex polysaccharides, production of antibacterial molecules (eg., defensins), and development of immune tolerance and resultant avoidance of allergies [26]. Other published reports suggest that specific species of Bacteroides, have a role in preventing infections caused by C. difficile [27,28]. In the gut, Bacteroides participate in carbohydrate fermentation which results in the production of volatile fatty acids (eg. short chain fatty acids, SCFA). These fatty acids are reabsorbed by the host through the large intestine and used as an energy source, thereby providing a substantial proportion of the daily energy requirement [29]. Additionally, Bacteroides benefit the survival of other intestinal bacteria that lack sugar utilizing enzymes.
Some previous studies have suggested that Bacteroides spp., especially B. vulgatus and B. fragilis, might play a role in the pathogenesis of ulcerative colitis (UC) [30-32]. Additionally, enterotoxigenic B. fragilis (ETBF) has been implicated in IBD, with the prevalence of the enterotoxin gene reported to be higher in luminal washings of patients with diarrhea compared to control group without diarrhea. It has been suggested that colonization with ETBF strains could lead to acute/chronic intestinal inflammation [33]. Further, B. fragilis has been shown to be responsible for a greater proportion of the bacterial mass in patients with IBD compared with controls [34].
The genus Clostridium has been associated with a number of GI issues, particularly diarrhea, constipation, vomiting, and nausea, characterizing irritable bowel syndrome (IBS) [35,36]. The impact of C. difficile infection on the healthcare system is large and becoming more prominent. For example, hospitalization due to C. difficile have increased by 237% since 2000, with an estimated in-hospital mortality of 4% [37,38].
Significantly, recent studies are beginning to show that probiotics have utility to treat Clostridium-associated gastrointestinal disturbances, including diarrhea. For example, S. boulardii has been shown to prevent or shorten the duration of antibiotic-associated diarrhea, and to prevent further recurrence of C. difficile-associated diarrhea [39].
The genus Roseburia is composed of Gram-positive anaerobic bacteria comprising 5 species including, Roseburia intestinalis, R. hominis, R. inulinivorans, R. faecis, and R. cecicola. They are commensal bacteria producing SCFA, especially butyrate, which are anti-inflammatory and help to maintain immunity. Studies have shown that an animal-based diet decreases the levels of Firmicutes species, including Roseburia, that metabolize dietary plant polysaccharides [40,41]. Further, human trials assessing the effect of weight-loss diets that are low in total carbohydrate also showed a decrease in the levels of fecal SCFA levels (eg., butyrate), correlating with a decrease in butyrate-producing bacteria such as Roseburia [42,43]. In this regard, even though the mycobiome diet is low in refined carbohydrates, we did not see a reduction in the level of Roseburia, but rather an increase, indicating that the mycobiome diet may be more beneficial than diets specifically formulated for weight loss due to its inclusion of resistant starches [44].
F. prausnitzii is a butyrate producer and has shown anti-inflammatory effects in both in vitro and in vivo mouse colitis models. Reduction in the Firmicutes species F. prausnitzii has been well documented in patients with CD, particularly those with ileal CD. In contrast, high levels of F. prausnitzii are found in healthy subjects [45]. For example, in our study with Crohn’s disease, we showed that the abundance of F. prausnitzii was higher in healthy relatives compared to patients with Crohn’s [11]. Therefore, modulation of F. prausnitzii levels could have preventive or therapeutic utility.
Our study showed that adherence to the mycobiome diet led to shifts in the bacterial and fungal gut communities of participants, with an increase in abundance of beneficial fungi and bacteria and a corresponding decrease in harmful microorganisms. Overall, this shift was associated with improved GI symptoms, weight loss, less fatigue, more energy, better sleep, and fewer cravings for empty-calorie foods.
Citation: Ghannoum M, Smith C, Adamson E, Isham N, Salem I, Retuerto M (2020) Effect of Mycobiome diet on Gut Fungal and Bacterial Communities of Healthy Adults. J Prob Health. 8:215. DOI: 10.35248/2329-8901.20.8.215
Received: 08-Dec-2019 Accepted: 02-Jan-2020 Published: 10-Jan-2020 , DOI: 10.35248/2329-8901.20.8.215
Copyright: © 2020 Ghannoum M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.