Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Short Communication - (2022)Volume 13, Issue 5
In analytical chemistry the Method development stands for the set of the experiments which is used for the proper analysis of the sample. It is used to identify, quantify and separate the compounds present in the sample. Various analytical instruments are used for these kinds of the studies [1]. But in most of the cases HPLC Analytical Method Development is preferred. The High Performance Liquid Chromatography (HPLC) is the separation of substances in a mixture is based on the rates at which they are dispersed across two phases, one of which is stable (stationary phase) and the other of which moves moving phase (mobile phase). It is the widely used analytical technique which is used for the method development and validation of the samples (Figure 1) [2].
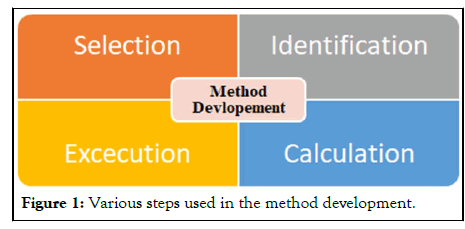
Figure 1: Various steps used in the method development.
Selection
In this approach to HPLC column selection, the bonded phase chemistry of the column is chosen on the basis of an analysis of the sample component structures. The physics of the column is chosen according to an analysis of the goals for the separation method.
Identification
HPLC analysis can be used for identification of compounds using Reference compounds by comparing their RT; this identification is confirmed by Spiking technique spiking the standard reference to the extract to indicate the accurate RT of peak in the extract.
Execution
1. For setting up the HPLC machine.
2. Make sure you have all your buffers set up.
3. Open the purge valve and purge the system for 5 minutes.
4. Add your samples into the auto sampler tray.
5. Stop the purge.
6. Close the purge valve.
7. Run the system at a normal flow rate (1 ml/min) with your buffer to equilibrate the column for 10 minutes.
Calculation
In this the various formula related to the calculation of results in the HPLC is used.
It is widely used because HPLC is unmalicious and may be used on thermally liable substances, it is more widely used. All the analytical, bio-analytical and natural compounds will be separated on HPLC. All the results are reproducible. HPLC also offers a diverse range of detection options due to the large number of different detectors available [3].
Validation is defined as the process of providing documented evidence that an instrument, a system, a method, a product, or a procedure performs as expected within specified design parameters and requirements so that the results obtained are reliable. Validation efforts should be broken down into separate components addressing the equipment both the instrument and the computer controlling it and the analytical method run on that equipment. After these have been verified separately, they should be checked together to confirm the system’s expected performance limits System Suitability Testing (SST). Illustrates the basic validation steps with regard to computerized HPLC equipment. The need for validation in analytical laboratories may originate from regulations such as Current Good Manufacturing Practices (cGMP), Good Laboratory Practice (GLP), Good Clinical Practices (GCP), and quality and accreditation standards such as the International Standards Organization (ISO) 9000 series. The various method validation parameters are used to evaluate whether the method is validated or not (Figure 2).
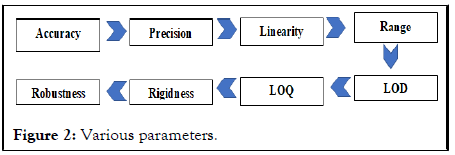
Figure 2: Various parameters.
The Validation Parameters is crucial for 2 primary reasons; it would be impossible to establish that the output being created is consistent and meets the present requirements. To ensure competence for use in the pharmaceutical sector.
1. Validation is considered as an integral part of the qualitycontrol system.
2. Assay validation is required by Current Good Manufacturing practices (CGMP) regulations [4].
HPLC method validation
In recent years, research literatures on the application of analysis techniques have included experimental designs for technique optimization as well as method validation. When evaluated through analytical technique, many validation parameters, including robustness, ruggedness and System Suitability Test (SST) were shown to provide more valid findings. It is critical to determine how robust or rugged a new approach is when it is refined. However, the use of the terminology robust/ robustness and rugged/ ruggedness for the description of analytical procedures is widely misunderstood in most literatures. Analytical technique is clearly an area with bright prospects for the future of analytical chemistry. Some of the most important features of chemometrics, as well as their applications in HPLC method development and validation [5].
Validation of HPLC instrumentation
Equipment validated under a specified set of conditions, must be revalidated whenever a change is made in those conditions. For example, changes in application software may influence the correct functioning of the computer system. The main qualification tests applied to HPLC instrumentation concern: The injection system. Parameters that have to be checked are precision and accuracy. The pump Parameters that have to be checked are flow rate precision and flow rate accuracy and stability regarding an isocratic pump. The detector Parameters that have to be checked are: wavelength accuracy detection, refractive index sensitivity, linearity, spectral quality (PDAD) short/long term noise, drift, and flow sensitivity. The first step is to establish internal specifications and then compare results obtained towards manufacturer ‘specifications and finally, interpret the differences. Within-day and inter-day reproducibility is the minimum test for routine use. Column plate number, N, is the most common parameter to describe the shape of the peak. Resolution Rs, is the measure of separation of two peaks, as well as of the efficiency of the column [6].
Chromatographic systems should be tested for suitability to the performance criteria of the method on a daily basis before and during routine application. An HPLC system should be tested before each single sample analysis or, if a series of samples are analyzed within a sequence, before each sequence. Retention time, peak height/area, resolution, and peak shape are the data used to monitor the performance of the HPLC system. Peak shape is one of the first things that experienced chromatographers notice when looking at a chromatogram. Ideal chromatographic peaks are Gaussian shaped but, in practice, most peaks show some peak tailing, which is measured using the asymmetry factor or the United States Pharmacopoeia tailing factor.
[CrossRef] [Google Scholar] [PubMed]
Citation: Kumar R, Chawla PA (2022) Discussion on Analytical Method development and Validation in HPLC. J Chromatogr Sep Tech. 13: 485.
Received: 25-May-2022, Manuscript No. JCGST-22-17632; Editor assigned: 30-May-2022, Pre QC No. JCGST-22-17632(PQ); Reviewed: 15-Jun-2022, QC No. JCGST-22-17632; Revised: 23-Jun-2022, Manuscript No. .JCGST-22-17632 (R); Published: 30-Jun-2022 , DOI: 10.35248/2157-7064.22.13.485
Copyright: © 2022 Kumar R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.