
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2025)Volume 15, Issue 3
Background: This study was designed to describe Bone Turnover Marker (BTM) profiles in women.
Patients and methods: In all, 197 patients (age 61 (36-90) years) comprising five groups were studied: Osteoporosis with (OPBP+) or without (OPBP-) bisphosphonate use; bone metastatic breast cancer with (BMBP+) or without (BMBP-) BP use; and healthy controls without BP (CBP-) use. Procollagen type 1 amino-terminal Propeptide (P1NP) and Carboxy-terminal Telopeptideof type 1 collagen (CTX) wereanalyzed.
Results: The medians (25%-75%; ng/mL) for P1NP were as follows: BMBP (236.95(165.0-328.0))> CBP(47.25(33.5-63.7))=OPBP-(50.9(37.4-63.9))>BMBP+(26.9(11.8-46.3))=OPB+(19.5(12.6-27.3)). The medians (25%-75%; ng/mL) for CTX wereas follows: BMBP-(0.567(0.457-0.803))=OPBP-(0.360(0.318-0.650))>CBP-(0.297(0.203-0.402))>BMBP +(0.101(0.052-0.202))=OPBP+(0.141(0.047-0.186)).
Conclusion: P1NP>145 ng/mL completely differentiated those with BMs. CTX<0.200 ng/mL differentiated those using BPs.
Breast cancer; Osteoporosis; Bone metabolism; Bone turnover markers
Bone Turnover Markers (BTMs) are substances released during the processes of bone resorption and formation that can be measured in blood or urine. There are many different markers, and Carboxy-terminal Telopeptide of type 1 collagen (CTX) and serum Procollagen type 1 amino-terminal Propeptide (P1NP) are the most indicated to measure resorption and formation, respectively. However, standardizing their use in real life still presents difficulties [1]. We intended to contribute preliminary data on the behavior of BTMs in two distinct clinical situations: Postmenopausal Osteoporosis (OP) and breast cancer with Bone Metastasis (BM). In this study population, CTX and P1NP demonstrated different abilities to distinguish the suppressive effects of Bisphosphonates (BPs) and the presence of BM [2].
This cross-sectional study was originally planned to investigate the prevalence of osteonecrosis of the jaw in women taking BPs to treat BM or OP published elsewhere. The BTMs CTX and P1NP were measured in a subgroup, and due to interesting results, we later compared our results with different samples of populations of women who took part in other research projects; the BTMs were measured with the same methods and in the same research laboratory [3].
Female patients from the following 3 institutions were included in this study: Sao Paulo school of medicine (UNIFESP)-Endocrinolgy and gynecology departments, women's treatment reference center-hospital Perola Byington (CRSM) and Paulista Center of Oncology (CPO), Sao Paulo, Brazil. This study protocol was reviewed and approved by the UNIFESP ethics committee (No. 0351/07), and informed consent was obtained from all individual participants included in the study [4].
Eligibility criteria
Women with a diagnosis of OP or BM as well as healthy patients were included in the study. Were included on the study patients who had clinical conditions. The first 50 patients from each group were randomly invited for the collection of morning fasting blood samples for biochemical analysis. The five groups studied were patients with OP using BPs (OPBP+, n=40), those with OP not using BPs (OPBP-, n=22), those with BM using BPs (BMBP+, n=44), those with BM not using BPs (BMBP-, n=6) and healthy controls not using BPs (CBP-, n=85) [5].
Fasting blood samples were collected from 197 patients in the morning. All samples were centrifuged and frozen immediately after collection. The bone formation marker P1NP (1.8% and 2.7% intra-and inter assay CVs, respectively) and the bone resorption marker CTX (4.6% and 4.7% intra-and inter assay CVs, respectively) were determined by electro chemiluminescence (Roche diagnostics, Indianapolis, IN, USA). All dosages were determined at an endocrinology laboratory and at the central laboratory of UNIFESP [6].
Statistical analysis
The Kolmogorov-Smirnov test was used to verify the normality of the data. For these results, we applied the nonparametric kruskal-wallis test with multiple tukey comparisons to verify the differences between groups [7].
As expected, the mean ages of the subjects were different. Those with BM were younger than those with OP (P<0.001). The mean ages of the CBP- group did not differ from those of the BMBP+ group, while women in the BMBP-group were the youngest (Tables 1-4) [8].
| Age (years), median (min-max) | 25% | Median (50%) | 75% | Min | Max | |
|---|---|---|---|---|---|---|
| OPBP+ | 74 (50-87) | 12.65 | 19.47 | 27.32 | 5 | 59.62 |
| BMBP+ | 63 (36-90) | 14.81 | 26.87 | 46.32 | 5 | 147.9 |
| BMBP- | 48 (46-68) | 165.63 | 236.95 | 328.25 | 146.8 | 388.4 |
| OPBP- | 70 (52-82) | 37.38 | 50.89 | 63.93 | 21.73 | 78.8 |
| CBP- | 58 (48-80) | 33.52 | 47.25 | 63.66 | 16.86 | 140.2 |
Table 1: Description of medians, different percentiles, and maximum and minimum P1NP values (ng/mL) in all studied groups.
| (P) | OPBP+ | BMBP+ | BMBP- | OPBP- |
|---|---|---|---|---|
| BMBP+ | 0.055 | 0.999 | ||
| BMBP- | <0.001*** | <0.001*** | ||
| OPBP- | <0.003** | 0.601 | <0.001*** | |
| CBP- | <0.001*** | 0.13 | <0.001*** | |
| Tukey 2/2 | Statistically significant | *0.05 | ||
| **0.01 | ||||
| ***0.001 | ||||
| OPBP+ = BMBP+ < OPBP- = CBP- < BMBP- | ||||
Table 2: The five groups shows statistically significant values.
| Age (years), median (min-max) | 25% | Median (50%) | 75% | Min | Max | |
|---|---|---|---|---|---|---|
| OPBP+ | 74 (50-87) | 0.077 | 0.141 | 0.186 | 0.01 | 0.444 |
| BMBP+ | 63 (36-90) | 0.052 | 0.101 | 0.202 | 0.01 | 0.479 |
| BMBP- | 48 (46-68) | 0.457 | 0.567 | 0.803 | 0.195 | 0.895 |
| OPBP- | 70 (52-82) | 0.318 | 0.36 | 0.65 | 0.305 | 0.853 |
| CBP- | 58 (48-80) | 0.203 | 0.297 | 0.402 | 0.032 | 0.892 |
Table 3: Description of medians, different percentiles, and maximum and minimum CTX values (ng/mL) in all studied groups.
| (P) | OPBP+ | BMBP+ | BMBP- | OPBP- |
|---|---|---|---|---|
| BMBP+ | 0.999 | <0.001*** | ||
| BMBP- | <0.001*** | <0.001*** | ||
| OPBP- | <0.001*** | <0.001*** | 0.547 | |
| CBP- | <0.001*** | <0.001*** | <0.001*** | |
| Tukey 2/2 | Statistically significant | *0.05 | ||
| **0.01 | ||||
| ***0.001 | ||||
| OPBP+ = BMBP+ <CBP- = OPBP- <BMBP- | ||||
Table 4: Statistically significant values of the five groups.
The presence of BMs was better identified by P1NP measurements. The median P1NP of the BMBP- group was approximately 5 times higher than the medians of the OPBPand CBP- groups. When compared with the groups using BPs, this difference was even greater, reaching approximately 10 times greater than those of the OPBP+ and BMBP+ groups (Figures 1 and 2). P1NP values above 146.8 ng/mL seemed to discriminate the presence of BMs before the use of BPs with high accuracy [9].
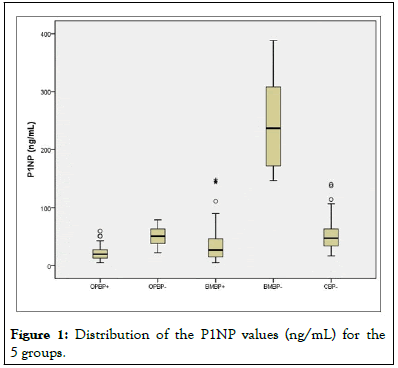
Figure 1: Distribution of the P1NP values (ng/mL) for the 5 groups.
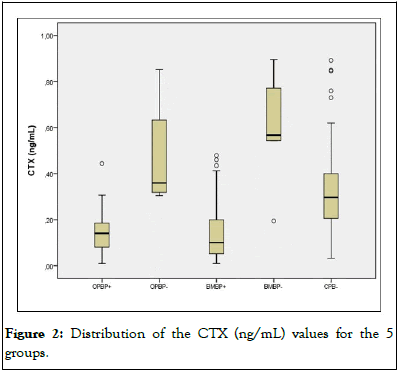
Figure 2: Distribution of the CTX (ng/mL) values for the 5 groups.
CTX concentrations, however, were more discriminatory for the suppressive effects of BPs on bone resorption. We observed that the patients using BPs (OPBP+ and BMBP+) had similar medians for CTX but had lower concentrations than the subjects in the CBP- and BMBP- groups. In all, 75% of the patients who used BPs in both groups had a CTX<0.2 ng/mL. However, there was an overlap of CTX values among the women who did not use BPs (the BMBP-, OPBP-and CBP-groups) [10].
Although BTMs have achieved great reproducibility and accuracy, there is still a lack of standardization and interpretation of these results in different clinical scenarios, such as for the screening of BMs. In the context of OP, the international osteoporosis foundation has proposed that P1NP and CTX are among the BTMs of choice for monitoring treatment [11].
Our results suggest that the bone resorption marker CTX is more suitable for evaluating the use of potent antiresorptives, such as BPs, and 75% of the women using BPs had CTX concentrations below 0.200 ng/mL. However, there was great overlap in these concentrations in women who did not use BPs, both in those with BMs and in women with OP or normal controls. P1NP concentrations, however, were not sensitive enough to identify women using suppressive therapy with BPs, showing great overlap with women with OP or normal controls. Some authors have suggested that BTMs should be used to monitor treatment, ideally in women with OP, with baseline samples being collected at baseline and after 3 and 6 months. In other studies, and in ours, CTX was not able to assist in the diagnosis or monitoring of BMs. In our study, it was more related to the discrimination of the use of BPs than to the pathology itself [12].
On the other hand, P1NP proved to be quite discriminatory for identifying women with BMs not using BPs. In our small sample, we observed that concentrations above 145 ng/mL almost perfectly differentiated the presence of BMs before the start of therapy with BPs. We recognize that our sample is quite small, and we also did not have access to baseline concentrations of these markers before the appearance of metastases. However, our preliminary results were very promising and suggest that, in longitudinal follow-up, the elevation of P1NP could indicate the appearance of early BMs, which requires a prospective assessment in a larger population of women with breast cancer [13].
Our data are in line with those described by who also found similar P1NP values (150 ng/mL to 240 ng/mL), and in the P1NP median was found to be 134 ng/mL. The BM group had a higher P1NP than the healthy control group and a group of breast cancer patients without metastases (70 ng/mL-120 ng/mL). Notably, these two groups had very similar P1NP values, highlighting that a significant change in the marker actually occurs in the presence of BMs [14].
Importantly, the levels of BTMs can vary with age, fasting, collection time, diseases and associated medications. Pre analytical care, as well as careful clinical evaluation, are fundamental for good accuracy and interpretation of the results. What we cannot ignore is the bone microenvironment of metastatic breast cancer, which has an important interaction between tumor cells [15]. The patients were separated into groups according to only the underlying pathology and use or nonuse of BPs; other medications and systemic changes were not exclusive factors for the study, providing a more reliable analysis of clinical practice [16].
BPs and other bone-modifying agents are drugs frequently used in patients with OP and BMs, and their use considerably alters the values of BTMs by reducing their values differentially [17]. We found that the P1NP and CTX values were approximately 2.5 times higher in patients with OP who did not use BPs. In cancer patients, the values of P1NP and CTX were 9 and 7 times lower, respectively, in patients who used BPs compared to those in patients who did not use BPs. In our study, we cannot conclude which periodicity should be checked for BTM values as predictors of pathology. Varsakian suggested monitoring from baseline and at 3 and 6 months to observe the effectiveness of inhibition of bone turnover and the consequent relationship with the pathology. Observed changes in BTM values after 3 months of discontinuing the use of BPs in their study [18].
The present study clearly demonstrates the difference between the values of the BTMs CTX and P1NP between healthy groups and patients who use or do not use BPs for different diseases, showing the interference of BPs in the changes in the BTM values. A P1NP>145 ng/mL completely differentiated the group of women with BMs from breast cancer not using BPs, which suggests that P1NP is better than CTX for discriminating BMs in patients who are not using BPs. CTX better discriminated patients who used BPs.
The levels of the BTMs CTX and P1NP should be considered complementary in the diagnosis and monitoring of bone diseases. Furthermore, it is essential to evaluate the medications used to treat patients since they can interfere with these values. More studies are needed to define the real role of bone metabolism markers in the diagnosis and monitoring of osteometabolic diseases.
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Research Ethical Committee of the Escola Paulista de Medicina–UNIFESP and Hospital Pérola (0351/07).
All participants included in the study provided written informed consent.
The authors declare no competing interests.
ALS and MLC designed the study, performed the experiments, analyzed the data and wrote the manuscript.
The other authors designed the study.
Not applicable.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Soares AL, Vieira JGH, Moreira LDF, Silva AGD, Castro ML, Simon SD, et al. (2023) Differential Behavior of Bone Turnover Markers in Women with Bone Metastatic Breast Cancer or Osteoporosis. J Clin Trials. 13:271.
Received: 11-Jan-2023, Manuscript No. JCTR-23-21375; Editor assigned: 13-Jan-2023, Pre QC No. JCTR-23-21375 (PQ); Reviewed: 27-Jan-2023, QC No. JCTR-23-21375; Revised: 11-Apr-2023, Manuscript No. JCTR-23-21375 (R); Published: 18-Apr-2023 , DOI: 10.35248/2167-0870.25.15.587
Copyright: © 2023 Soares AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.