Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2023)Volume 14, Issue 4
Introduction: Measurement of urinary excretion of corticosteroids and their metabolites is used to evaluate adrenal function in Cushing's syndrome or adrenal cancer. Quantification of Urinary Free Cortisol (UFC) is recommended for Cushing’s syndrome diagnosis.
Objective: To develop an easy, reproducible and specific assay technique to quantify UFC by High Performance Liquid Chromatography (UHPLC)
Methods: We developed a method based on an Ultra-High Performance Liquid Chromatography (UHPLC) assay coupled to a single Quadrupole mass (QDa) detector. Samples were extracted by Supported Liquid Extraction (SLE). Urines were pre-treated with 1% formic acid and the internal standard. Cortisol was eluted with dichloromethane. Solvent was evaporated and samples were reconstituted with water. The compounds were separated on a HSS T3 column with a gradient elution (water, 0.1% formic acid and acetonitrile). Injection volume was 1 µl and the flow rate 0.6 ml/min. We investigated the method regarding linearity, precision, recovery, accuracy and limits of detection and quantification.
Results: The linear range for this method was from 5 μµg/l to 1000 µg/l with a determination coefficient of 0.99. The precision was evaluated by the Relative Standard Deviation (RSD) for intra-assay and inter-assay and was below 9% for both of them. The method showed adequate cortisol recovery (>91%). The limit of detection was 0.28 µg/L and the limit of quantification was 0.75 µg/L.
Conclusion: The UFC UHPLC-QDa detection method with a SLE step shows adequate performances. It is easy, reproducible and suitable for routine laboratory use.
Cushing’s syndrome; Urinary free cortisol; Mass detection; Supported liquid extraction; Ultra-high performance liquid chromatography
Cortisol (hydrocortisone) is the main and most abundant glucocorticoid hormone synthesized from cholesterol by a multienzyme cascade in the adrenal glands [1,2]. Cortisol secretion is subjected to a nycthemeral rhythm, its blood concentration being maximum in the morning between 7 and 10 am and minimum at midnight. This secretion is regulated by the Hypothalamo-Hypophyso-Adrenal (HHA) axis. Cortisol plays a critical role in controlling protein, lipid and carbohydrate metabolism, regulating blood pressure, fighting infections and in the body's response to stress.
In the blood, circulating cortisol is mainly bound to transcortin or Cortisol Binding Globulin (CBG). Only the free cortisol is biologically active (<10%) [3]. Urinary Free Cortisol (UFC) correlates well with the plasma free cortisol concentration. The cortisol quantification is recommended to confirm hypercorticism, such as Cushing's syndrome, and hypocorticism, such as Addison disease which is an adrenal insufficiency [4,5].
Cushing's syndrome results from chronic and excessive circulating levels of glucocorticoids. Biochemical tests are needed to confirm the clinical suspicion. The three first-line screening tests recommended are a measurement of 24-hours UFC, a low- dose dexamethasone suppression test, and a midnight serum or salivary cortisol measurement [6-10]. UFC is non-invasive and unlike plasma cortisol secretion, it smoothes out the nycthemeral variation. The test has a reported sensitivity of 95%-100% and a specificity of 94-98% for the diagnosis of Cushing’s syndrome. A very high level of UFC negates the need for other test procedures in patients with obvious symptoms and signs of Cushing’s syndrome.
Wood and al showed that immunoassays have significant positive analytic bias compared to HPLC due to antibody cross-reactivity in urines with cortisol metabolites and synthetic glucocorticoids [7]. These cross reactions lead to an overestimation diagnosis of hypercorticism. High-Performance Liquid Chromatography (HPLC) can separate interfering substances and gives a more specific measurement of urinary cortisol [11-14].
The aim of this study was to develop an easy, rapid and sensitive UFC UHPLC method connected with a mass detector and using Supported Liquid Extraction (SLE) as a clean-up procedure. SLE is a cartridge-based variant of Liquid-Liquid Extraction (LLE) in which extraction is achieved by passing a water immiscible organic mobile phase through an aqueous stationary phase formed on an inert diatomaceous earth or a synthetic support. Compared to LLE, SLE’s support allows an intimate contact between the aqueous and the organic phases, possibly leading to analyte recoveries higher than the ones obtained with LLE [15-19]. SLE avoid the delicate steps as the phases separation, but also the emulsion present in the classic LLE method and is efficient with less volumes of solvents [13]. Although the standard for urinary free cortisol is LC-MS/MS, the cost equipment and the easy and reproducible use of the mass detector (QDa, Waters) make it an interesting option for clinical laboratory.
Institutional Review Board (IRB)/Ethics Committee ruled that approval was not required for this study.
Solutions preparation
Patient’s 24-hour urines were frozen immediately after collection. After the thawing at room temperature the day of analysis, 200 µl of urine, standard or control were mixed with 20 µl of 10% formic acid, 40 µl of 10 µg/ml internal standard, and 140 µl of water.
Cortisol solution
A 50 µg/ml stock solution of cortisol was obtained by diluting 5 mg of hydrocortisone (Infinity Pharma Lot: 20E14-B11-201954) in 100 ml of water. We have chosen a commonly prescribed drug for many reasons including administrative simplicity; ease of supply but also for its economic advantages.
Internal Standard (IS)
A 50 µg/ml methylprednisolone solution was obtained by diluting 5 mg of methylprednisolone (Sigma-Aldrich, Lot: LRAC4830, Saint- Louis, USA) in 100 ml of water. The solution was then diluted 1:5 in water to obtain the 10 µg/ml working solution.
UHPLC controls and standards preparation
Two levels of quality control corresponding to physiologically low and high cortisol concentrations (20 and 150 µg/l), were prepared each day of analysis from the cortisol solution. Six standards (5, 10, 50, 100, 250, 500 µg/l) were prepared from the cortisol solution by dilutions in water.
Supported liquid extraction
The pretreated samples were extracted by supported liquid extraction using a 400 µl Novum 96-Well plate (Phenomenex, lot S19-002470, Torrance, California). A drying time of 5 min was waited after loading. The elution was performed with 2 × 900 µl of dichloromethane (Biosolve, lot 10042731, Dieuze, France). The collected eluate was then evaporated to dryness at 40°C for 75 min (SPD121P-230, ThermoScientific USA) and reconstituted in 100 µl of water for 5 min on a centrifuge.
Chromatographic conditions
The chromatographic conditions were optimized using an Ultra- High Performance Liquid Chromatography (UHPLC) system coupled with a QDa detector (Acquity, Waters, Milford, USA). AHSS T3 pre column (Acquity UPLC® HSS T3 1,8 µm VanGuard™ Pre-Column 2.1 × 5 mm, lot 0216390091, Waters) and a HSS T3 reverse phase column (Acquity UPLC HSS T3 1.8 µm 2.1 × 50 mm, lot 0213382772, Waters) were used as stationary phase. Separation was achieved with an acid mobile phase consisting of water with 0.1% formic acid (Biosolve, lot 1198871, Dieuze, France) and acetonitrile (Biosolve, lot 1387001, Dieuze, France) that was used in gradient at a flow rate of 0.6 mL/minute. The run time was set to 6 minutes (t=0 minute: 20% acetonitrile; t=2 minutes: 35%; t=3 minutes: 95%; t=5 min 20%). The column temperature was maintained at 40°C. The injection volume was1 µl. Ionization was in positive mode. The capillary voltage was set to 1 kV and the probe temperature was held at 600°C. Cortisol (m/z: 363.2 Da) and methylprednisolone (IS) (m/z: 375.2 Da) were detected in Single Ion Recording (SIR) mode with a cone voltage of 10 V. Data was acquired with the software Empower 3 (Waters). The sampling rate was 2 points per second. Cortisol peak and methylprednisolone peak were both detected with a retention time of 1.9 min and 2.4 min.
Method validation
Linearity: The linearity was evaluated by diluting a 1000 µg/l hydrocortisone solution in water (1, 5, 10, 50, 100, 250, 500, 1000 µg/l). 200 µl of each dilution underwent the extraction step and were analyzed just as the samples.
Precision: The precision of the method was checked by analyzing 20 measurements of 2 hydrocortisone concentration levels (20 and 150 µg/l) within-day (repeatability) and between-day (intermediate precision). These solutions corresponded to control solutions. The precision was expressed by the Relative Standard Deviation (RSD).
Accuracy: The accuracy was evaluated by calculating the bias between the mean of results obtained in the inter-assay experiment and the target value. It was calculated for two levels of concentration (20 and 150 µg/l).
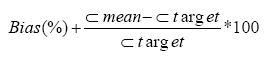
Limits of detection and quantification: The Limit of Detection (LOD) and Limit of Quantification (LOQ) were obtained by injecting 10 blanks (purified water) and quantifying the background noise at the retention time of cortisol. LOD and LOQ were calculated as follows:
LOD=mean+3*Standard Deviation (SD)
LOQ=mean+10*Standard Deviation (SD)
Trueness: The trueness was evaluated by submitting our samples to externalized quality control testing.
Recovery: The recovery percentage was assessed by spiking 3 patient’s urines with amounts of cortisol (10, 50 and 100 µg/l). Samples were extracted and the observed concentrations were compared to the expected values.
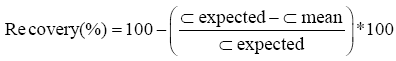
Carry-over: Carry-over was evaluated by injecting 3 high concentrations of cortisol (H) (500 µg/l) followed by 3 low concentrations of cortisol (L) (5 µg/l).
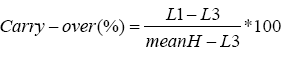
Robustness: To test the robustness of the method, small variations in method parameters were performed. The extraction and the injection of the 6 standards and the 2 controls were carried out with:
• 3 evaporation durations: 70, 75 and 80 minutes.
• 3 reconstitution durations: 3, 5 and 10 minutes.
• 3 mobile phases (variation of the aqueous phase): 0.98, 1 and 1.2% formic acid.
Chromatographic conditions determination
Examination of cortisol and methylprednisolone (IS) structures suggested a reverse phase analysis given that molecules were both hydrophobic (log P: cortisol: 1,275, methylprednisolone: 1,525) thereby a HSS T3 1.8 µm column (Waters) was chosen. Separation was achieved using an acidic mobile phase composed of water with 0.1% formic acid and acetonitrile. Acidic pH allowed reproducible separation given that only one neutral species was in solution for both molecules, therefore avoiding secondary interactions with residual silanol groups. Using acetonitrile as organic phase gives lower retention times than those obtained with methanol since acetonitrile is a stronger solvent than methanol [20-22].
Different flow rates (0.3, 0.6 ml/min) and injection volume (1.5 µl) were tested before choosing parameters allowing good peak shapes without any tailing: 0.6 ml/min and 1 µl. The retention times of cortisol and IS were 1.9 and 2.4 minutes (Figure 1). The chromatographic method optimization was continued with the variation of the cone voltage (5, 10, 15, and 20 V) and the capillary voltage (0.8, 0.9 and 1 kV). QDa parameters producing greatest peak area for both cortisol and IS were: Cone voltage=10 V and capillary voltage=1 kV.
Figure 1: Cortisol (m/z: 363.2 Da) and methylprednisolone (375.2 Da) chromatograms.
Extraction method selection
Two extraction methods were tested: Liquid-Liquid Extraction (LLE) and Supported Liquid Extraction (SLE). LLE is a complicated technique, it induces an emulsion, requires different separation phases and more solvent than SLE therefore LLE was tested but excluded. SLE was tested on 96 well plates and easily and quickly produced a clear product ready for analysis without conditioning or equilibrating steps [15,16,19]. It allowed a good recovery rate and produced clean chromatograms. Interference assays were not tested given that interference risks were limited by using SLE, specific mass detection and specific retention time.
SLE optimization
Before being loaded on the SLE plate, samples have to be diluted 1:1 in aqueous solvent. For this purpose, water and 1% formic acid were tested. The acidic samples dilution provides higher intensity peaks than the ones obtained with water dilution (Figure 2). Elution was performed with dichloromethane and optimized by varying the evaporation time (70, 75, 80 min). Total organic phase evaporation was achieved after 75 minutes.
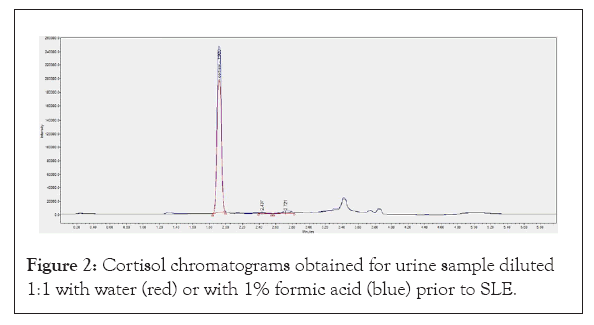
Figure 2: Cortisol chromatograms obtained for urine sample diluted 1:1 with water (red) or with 1% formic acid (blue) prior to SLE.
Validation of the method
Cortisol concentrations exhibited consistent linearity over a range from 5 µg/l to 1000 µg/l with a correlation coefficient of 0.99 (Figure 3). The lower limit of the linearity range was fixed at 5 µg/l, which is the lowest concentration giving a deviation percentage lower than 15% between the observed and the expected value. Calibration curves carried out each day of analysis were defined based on the clinical interest in the linearity range (5-500 µg/l). Random effects like different operators or analysis days were evaluated by the precision. Repeatability coefficient of variation was <1.24%. Intermediate precision coefficient of variation was <8.6% (Table 1). The bias between the mean of results obtained in the inter-assay experiment and the target value was <5.9%. The LOD was 0.28 µg/l and LOQ was 0.75 µg/l based on the background noise. The method showed adequate cortisol extraction recovery which was greater than 91%. No matrix effect was observed during recovery manipulation. The injection of 3 low concentration samples after 3 high concentration samples showed a carry-over lower than 0.05%. The quantification of UFC was not affected by small variations in method parameters given that results variation was <5.8% which is lower than the between-day variation. The trueness was assessed through external quality control testing. The difference between assays and target value was <15% (Table 2). Cortisol is a suitable marker for the diagnosis of Cushing’s syndrome. This analyte can be measured in several biological matrices (urine, blood, saliva) [1,21]. UFC is not influenced by the nycthemeral rhythm, so it offers an integrated index of steroid production over a period of 24 hours [23-28]. Various techniques allow the cortisol quantification including immunoassays and HPLC [11]. Chromatography is usually preferred because of its good specificity. Chromatography coupled with mass detector achieves high specificity and sensitivity of analysis [18]. Therefore a rapid, easy and reliable method using SLE followed by UHPLC system coupled with a QDa detector was established for the analysis of UFC.
| Concentration | Within-day precision (n=20) | Between-day precision (n=20) | ||||
|---|---|---|---|---|---|---|
| Mean (μg/ml) | SD (μg/ml) | RSD (%) | Mean (μg/ml) | SD (μg/ml) | RSD (%) | |
| Low concentration | 19,207 | 0,238 | 1,238 | 19071 | 1,638 | 8,587 |
| High concentration | 1,48,069 | 0,935 | 0,632 | 1,46,361 | 9.8 | 6.7 |
Table 1: Within-day and between day precision of the method.
| Test number | Results (µg/ml) | Target value (µg/ml) | Bias (%) |
|---|---|---|---|
| 22BS01 | 45,930 | 44,473 | 3,28 |
| 22BS02 | 6,31,968 | 5,96,376 | 5,97 |
Table 2: Trueness based on external quality controls.
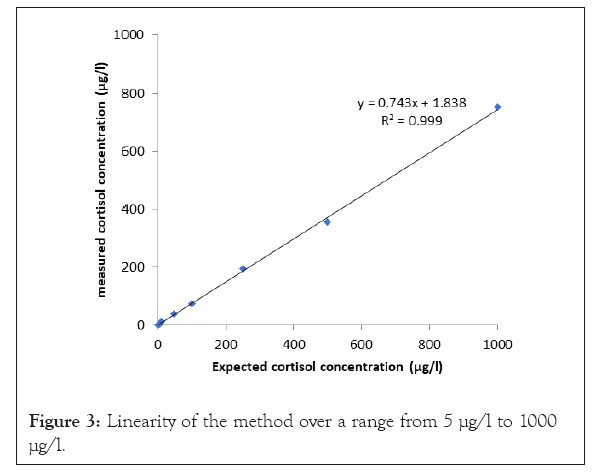
Figure 3: Linearity of the method over a range from 5 µg/l to 1000 µg/l.
SLE consists in a liquid-liquid extraction in the presence of a sorbent, it enables an efficient extraction with less organic solvent and without any emulsion formation compared to LLE [15,16,19]. The range of linearity was from 5 µg/l to 1000 μµg/l, allowing the quantification of high concentrations, typical of patients with Cushing’s syndrome, as well as low concentrations found in the normal population or in patient with Addison disease [4,5,18,28]. The developed method’s parameters are validated regarding that the precision of the cortisol quantification is 8.6%, the accuracy is 5.9%, the recovery is >91% and the carry-over is <0.05% [29]. The robustness is demonstrated given that results variation is <5.8% which is lower than the between-day variation. The trueness is also demonstrated given that the difference between assays and target value is <15%. We used methylprednisolone as internal standard to take UFC possible extraction loss into account. This approach allows good reproducibility. Methylprednisolone is synthesized from cortisol and has a similar structure as cortisol that can be seen in Figures 4 and 5. The intermediate precision of the UFC quantification with this developed method is <15% indicating that methylprednisolone is suitable as internal standard.
Figure 4: Structure of cortisol.
Figure 5: Structure of methylprednisolone.
This report describes the development of 24-hours urinary free cortisol quantification method by Ultra-High Performance Liquid Chromatography (UHPLC) coupled with a mass detector with a Supported Liquid Extraction as a clean-up procedure. The method is simple, cost-effective, quick, and reproducible. This method permits quantification of high concentrations of cortisol typical of Cushing’s syndrome as well as low concentrations found in patients with Addison disease. Its high specificity provides a low rate of sample interference by exogenous corticosteroids.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Citation: Alexe M, Quentin D, Nicolas G, Marie-Lise C, Melanie C (2023) Development and Validation of a Quantification Method of Urinary Free Cortisol in 24 hours Urine by Mass Detection, J Chromatogr Sep Tech.14:523
Received: 02-May-2023, Manuscript No. JCGST-23-23835 ; Editor assigned: 04-May-2023, Pre QC No. JCGST-23-23835 (PQ); Reviewed: 23-May-2023, QC No. JCGST-23-23835; Revised: 01-Jun-2023, Manuscript No. JCGST-23-23835 (R); Published: 09-Jun-2023 , DOI: 10.35248/2157-7064.23.14.523
Copyright: © 2023 Alexe M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.