Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research - (2022)Volume 13, Issue 1
An accurate and reproducible liquid chromatographic assay method was developed and validated for the determination of Pseudoephedrine HCL and Levocetirizine HCL in Tablet formulation. Acetonitrile and Water (50:50 v/v) was used for reverse-phase liquid chromatography at detection wavelength 230 nm to determine the contents of Pseudoephedrine HCL and Levocetirizine HCL in Tablet formulation. The method was validated by determining parameters such as specificity, linearity, LOD and LOQ, precision, accuracy, ruggedness and robustness. The method was found to be specific against placebo interference. Linearity was evaluated over the concentration range of Pseudoephedrine HCL 10-120.0 μg/mL and Levocetirizine HCL 0.5 to 12.0 μg/mL. The correlation of coefficient obtained was 0.998 for both. The intraday and interday precision values of the system and method was determined. The accuracy of the method obtained 99.98% for Psedoephedrine HCL and 100.50% for Levocetirizine HCL. The proposed method was found robust, while there was slight but deliberate changes were made in analytical conditions. The developed will be suitable for the assay of in raw materials, capsule formulation as well in other forms of combined dosage forms.
Pseudoephedrine HCL; Levocetirizine HCL; Internal standard; HPLC/UV; Method Development; Validation
The combined dosage forms of pharmaceuticals products will be for the synergistic effect or to give better effect than single drug. In this study Pseudoephedrine Hydrochloride-[1-(methyl-amino) ethyl]-[S-(R*,R*)]-benzene methanol OR (1S,2S)-2-methylamino-1- phenylpropan-1-ol hydrochloride. pseudo ephedrine only relieves nasal congestion commonly associated with colds or allergies. Antihistamines, which modify the systemic histamine-mediated allergic response [1].
Levocetirizine Hydrochloride (2-(4-[(R)-p-Chloro-α- phenylbenzyl]-1-piperazinyl)ethoxy) acetic acid. Levo-cetirizine is the R-enantiomer of cetirizine and is used similarly, as the hydrochloride, for the symptomatic relief of allergic conditions including rhinitis and chronic urticaria. The structures are as follows in Figure 1.
Figure 1:Structures of Levo-cetirizine hydrochloride and
Pseudoephedrine hydrochloride.
Note: (A) Levo-cetirizine Hydrochloride; (B) Pseudoephedrine
Hydrochloride
The combination of Pseudoephedrine HCL and Levocetirizine HCL tablet contains Levocetirizine Dihydrochloride 5 mg and Pseudoephedrine Hydrochloride 120 mg. The daily dose of Adult is one tablet only. This combination gives better effect and up to 24 hours.
A literature review reveals that only a few methods have been developed for the quantification of individual drugs like Pseudoephedrine HCL, Levocetirizine HCL [2,3].
HPLC instrumentation
The chromatographic separation was performed on the Waters liquid chromatographic system equipped with (Waters-1515) isocratic solvent delivery system pump with (Waters-2487) dual wavelength absorbance UV-detector and Rheodyne 7725i injector with 50 mL loop volume. Breeze 3.3 data station was applied for data collecting and processing. A phenomenox C18 column (25 cm × 4.6 mm i.d., 5 μm particle size) was used for the separation. The mobile phase consisted of a mixture of water and acetonitrile in the ratio of (50:50, v/v). The mobile phase was prepared daily, filtered, sonicated before use and delivered at the flow rate of 1.0 mL/min. The detection wavelength fixed at 230 nm an isobestic point for both drugs as shown in Figure 2.
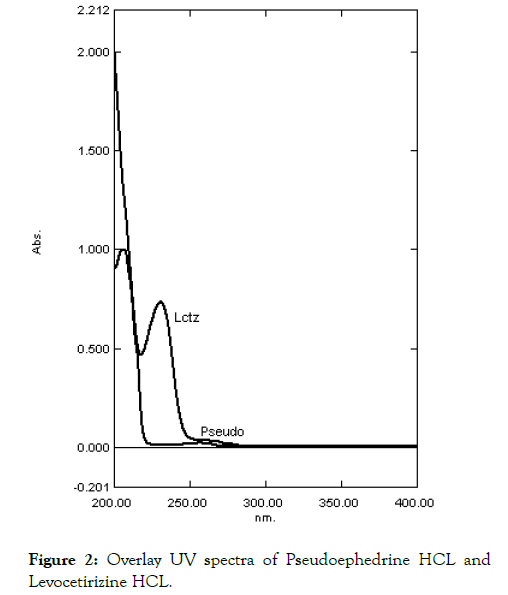
Figure 2: Overlay UV spectra of Pseudoephedrine HCL and Levocetirizine HCL.
Chemicals and reagents
The pharmaceutical grade gift reference and working standards of Levocetirizine di hydrochloride (99.55%), from from Apex pharmaceuticals (Medak, India) Pseudoephedrine hydrochloride (99.45%) was a gift sample from Avon organics Limited (Solapur, India). Internal standard Pantoprazole (99.18%) gift samples from Apex pharmaceuticals (Medak, India). The solvents Acetonitrile and Water used were of HPLC grade; all analytical grade chemicals and solvents were obtained from E Merck (India) Ltd, Mumbai. Water HPLC grade was obtained from a Milli-QRO water purification system Table 1 [4].
| Chromatographic Conditions | ||
|---|---|---|
| Solvent | Acetonitrile and Water | (50: 50 v/v) |
| λmax | Pseudoephedrine HCL | 256.6 nm |
| Levocetirizine HCL | 230.6 nm | |
| Linearity and range UV | Pseudoephedrine HCL | 10-60 µg/ml |
| Levocetirizine HCL | 2-10 µg/ml | |
| Linearity and range HPLC | Pseudoephedrine HCL | 10-80 µg/ml |
| Levocetirizine HCL | 5-40 µg/ml | |
Table 1: Chromatographic conditions Pseudoephedrine HCL and Levocetirizine HCL.
Procedure for UV calibration
The selection of wavelength is carried out and linearity is confirmed by UV-Spectrophotometry. Th wavelength selected for Pseudoephedrine HCL 256.6 nm and for Levocetirizine HCL 230.6 nm. The overlay spectra as shown in Figure 1 and linearity shown in Table 1. The linearity curve obtained as per shown in Figure 3.
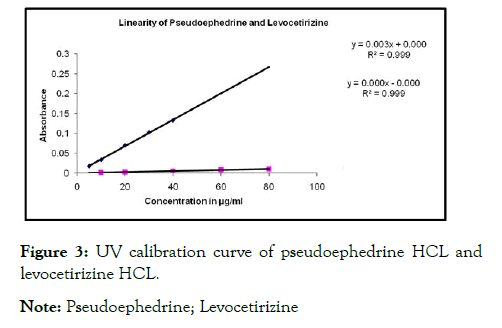
Figure 3: UV calibration curve of pseudoephedrine HCL and
levocetirizine HCL.
Note: Pseudoephedrine; Levocetirizine
Standard solutions and calibration curves by HPLC
Standard stock solutions were prepared at concentration of 1 mg/mL of Pseudoephedrine HCL and Levocetirizine HCL separately using a mixture of water and acetonitrile (50:50, v/v). The working standard solutions were prepared of different concentrations ranging from 10.0 to 60.0 μg/mL and 2.0 to 10.0 μg/mL for Pseudoephedrine HCL and Levocetirizine HCL respectively, by maintaining the concentration of Diclofenac Sodium (DS) at a constant level of 50 μg/mL. From the above each mixture 50 μL was injected in triplicate for the estimation of standard drugs, under the optimized chromatographic conditions, a steady baseline was recorded; the typical chromatogram was recorded for standard Pseudoephedrine HCL and Levocetirizine HCL with Pantaprazole internal standard as shown in Figure 4 [5]. The retention times of standard and internal standard were found at 2.0, 7.50 and 14.00 min, respectively. The calibration curve was obtained by simple linear regression of concentration of drug to the response factor as denoted in Table 2 [6].
| Pseudoephedrine | Levocetirizine | ||||||
|---|---|---|---|---|---|---|---|
| IS Peak area | Conc. µg/ml | Peak area | Response factor | IS Peak area | Conc. µg/ml | Peak area | Response factor |
| 10 | 435596 | 0.1439 | 0.5 | 11866 | 0.0038 | ||
| 20 | 565257 | 0.1825 | 2 | 52204 | 0.0168 | ||
| 40 | 752160 | 0.2429 | 4 | 109475 | 0.0353 | ||
| 3096533 | 60 | 970396 | 0.3085 | 3096533 | 6 | 175293 | 0.0566 |
| 80 | 1108912 | 0.3581 | 8 | 225019 | 0.0726 | ||
| 100 | 1273184 | 0.4111 | 10 | 296187 | 0.0956 | ||
| 120 | 1452614 | 0.4691 | 12 | 354580 | 0.1145 | ||
Table 2: Linearity of Pseudoephedrine and Levocetirizine by HPLC.
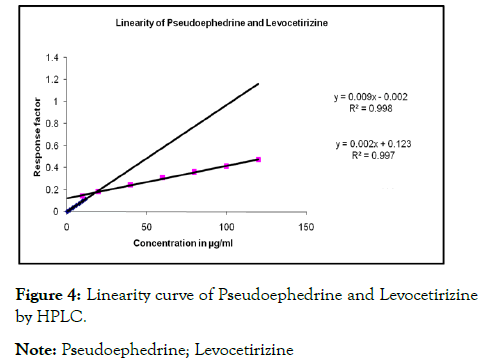
Figure 4: Linearity curve of Pseudoephedrine and Levocetirizine
by HPLC.
Note: Pseudoephedrine; Levocetirizine
Analysis of tablets by HPLC
Twenty tablets each containing 120 mg of Pseudoephedrine HCL and 5 mg of Levocetirizine HCL were weighed and the amount equivalent to one tablet content was weighed, powdered and dissolved in acetonitrile: water (50:50 v/v) with appropriate amount of IS was added in mixture. The drugs were extracted with same solvent mixture, filtered and further dilutions were made by mobile phase to get a concentration of 2 μg/ml of Pseudoephedrine HCL 40 μg/ml of Levocetirizine HCL and 50 μg/ml of as Pantaprazole internal standard [7,8]. These solutions were injected for the estimation. The retention times obtained for Pseudoephedrine HCL, Pantaprazole (IS), were Levocetirizine HCL at 2.01, 7.60 and 14.10 min respectively as in Figure 5.
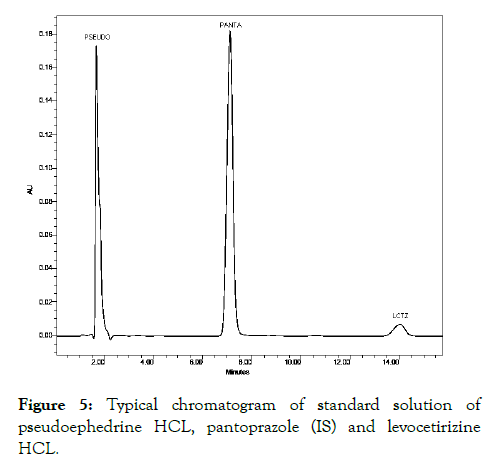
Figure 5: Typical chromatogram of standard solution of pseudoephedrine HCL, pantoprazole (IS) and levocetirizine HCL.
Method development
The aim of present work was to develop simple RP-HPLC with ultraviolet detection for the simultaneous determination of Pseudoephedrine HCL and Levocetirizine HCL in solid pharmaceutical dosage forms. As the solubility of Pseudoephedrine HCL and Levocetirizine HCL as sparingly soluble in water therefore mixture of acetonitrile and water (1:1, v/v) was used as solvent for preparation of all standard and sample solutions.
Several attempts were performed in order to get satisfactory resolution of Pseudoephedrine HCL and Levocetirizine HCL in different mobile phases with various ratios of organic phases and buffers by using C18 column. Initially the mobile phase used was mixture of water and methanol followed by water and Methanol in different ratios. Then Acetonitrile and Water in ratio of (80:20 v/v), (75:25 v/v), (50:50 v/v), mobile phase was used by isocratic elution to obtain satisfactory and good resolution with internal standard Pantaprazole. The effect of solvent composition by changing the ratio of acetonitrile-Water 50:50% v/v was shown satisfactory resolution. Therefore this method was sensitive to mobile phase ratio. The effect of change in pH of mobile phase by ± 0.2 does not shown significant change in retention time of each analyte. The retention time of Pseudoephedrine HCL and Levocetirizine HCL, Pantaprazole (IS) on C18 column was found satisfactory with above mobile phase at a flow rate of 1.0 mL/min. The retention times obtained for Pseudoephedrine HCL, Pantaprazole (IS), were Levocetirizine HCL at 2.01, 7.60 and 14.10 mins respectively as in Figure 6.The resolution of standard and sample solution found reproducible and satisfactory [9].
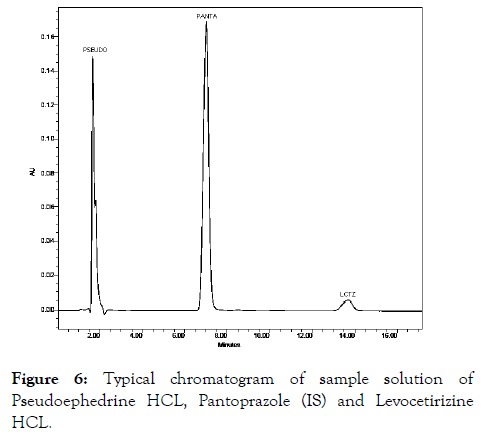
Figure 6: Typical chromatogram of sample solution of Pseudoephedrine HCL, Pantoprazole (IS) and Levocetirizine HCL.
Selection of UV wavelength and internal standard
The detector wavelength of the present study was selected on the basis of higher sensitivity. The internal standard was selected due to its suitable retention time, recovery and lack of interference with endogenous peaks and also not much affected by the mobile phase pH. These phenomena helped their good separation with other peaks.
Developed method validation
Linearity and range: The linearity and range HPLC method was determined at six concentration levels for ATV and FEB. The linearity and range of Pseudoephedrine HCL 10-120.0 μg/mL and Levocetirizine HCL, 0.5-12.0 μg/mL respectively. The calibration curve was constructed by plotting response factor against concentration of drugs. The slope and intercept values of calibration curve for Pseudoephedrine HCL y=0.002 X-0.123 (R2=0.997) and for Levocetirizine HCL y=0.009X-0.002 (R2=0.998) where Y represents the ratio of peak area ratio of analyte to IS and X represents analyte concentration [10].
Accuracy and precision: The accuracy of the developed method was determined using a mixture containing Pseudoephedrine HCL and Levocetirizine HCL, three concentration levels of standard drugs corresponding to 80%, 100% and 120% and determining the recovery of the added drug, at each concentration six determinations were performed.
The precision of the method was assessed by replicate analysis of pharmaceutical preparations. The precision and accuracy of HPLC method was obtained by analyze on the same day (intraday accuracy) and analyze on the different days by triplicate analysis (inter-day accuracy) and expressed as relative standard deviation percentage (R.S.D%). The correlation coefficient and the data of precision accuracy are reported in Table 3.
| Analytical methods | Detection wavelength/λmax (nm) | Linearity range | Formulations(mg)/dose | % Recovery (Mean ± S.D) n=3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo | Lctz | Pseudo | Lctz | Labeled amount pseudo | Amount found pseudo | Labeled amount Lctz | Amount found Lctz | Pseudo | Lctz | |
| HPLC method | 230 | 230 | 10-120 | 0.5-12 | 120 | 119.5 | 5 | 5 | 99.98 ± 1.68 | 100.50 ± 1.791 |
Table 3: Assay and %Recovery of Pseudoephedrine HCL and Levocetirizine HCL of Tablets.
LOD and LOQ: The sensitivity of Pseudoephedrine HCL and Levocetirizine HCL, was estimated as Limit Of Detection (LOD) and Limit Of Quantification (LOQ), they were calculated by the use of the equations LOD=3.3 × N/B and LOQ=10 × N/ B, where N is the standard deviation of the peak areas of the drugs (n=3), taken as a measure of the noise, and B is the slope of the corresponding calibration plot. The LOD and LOQ Values were reported in Table 4.
| S.No. | Parameters | Pseudoephedrine | Levocetirizine |
|---|---|---|---|
| 1 | Retention time (minutes) | 1.85 ± 0.04 | 14.10 ± 0.02 |
| 2 | Theoretical plates | 2457 | 8593 |
| 3 | Resolution | 24.1 | |
| 4 | Asymmetry factor | 0.96 | 1.01 |
| 5 | Calibration range (µg/ml) | 10-120 | 0.5-12 |
| 6 | Correlation coefficient (r) | 0.997 | 0.998 |
| 7 | LOD (ng/ml) | 135 | 14 |
| 8 | LOQ (ng/ml) | 412 | 42 |
Table 4: System suitability parameters of Pseudoephedrine HCL and Levocetirizine HCL.
Recovery and stability: The accuracy of the method was determined by the method of standard addition at three different levels. The recovery studies were carried out for capsules by spiking standard of each drugs equivalent to 80%, 100%, and 120% to the original amounts present in each drug formulations. The average recoveries were as reported in Table 4 [11].
In order to demonstrate the stability of both standard and sample solutions during analysis, both solutions were analyzed over a period of 24 h at room temperature. The retention time and peak area of Pseudoephedrine HCL and Levocetirizine HCL, remained almost similar (%R.S.D. less than 2.0) and no significant degradation within the indicated period, thus indicated that both solutions were stable for at least 24 h, which was sufficient time to complete the whole analytical process [12,13].
In conclusion, a novel HPLC method was developed and validated for the simultaneous determination of Pseudoephedrine HCL and Levocetirizine HCL, in solid dosage form. It assured the satisfactory precision and accuracy and has high analytical potential. The proposed method was found simple, accurate, economical and reproducible and can be applied for routine analysis in laboratories. RPHPLC method is suitable for the quality control of the raw materials, formulations, dissolution studies and can be employed for bioequivalence studies for the same formulation.
The author’s thank Hetero laboratories, Hyderabad for providing gift samples of Pseudoephedrine HCL from HAB Pharmaceutical & Research Ltd (Thane, India) and Levocetirizine HCL, M/s. Cadila Pharmaceuticals Ltd,Ankleshwer for providing a gift sample Pantaprazole (IS). The authors are also thanking Mr. Supe (Drug Inspector). The authors are grateful to “His Holiness Jagadguru Sri Sri Shivarathree Deshikendra Mahaswamigalavaru” of Sri Suttur Mutt, Mysore and AICTE (QIP) cell for providing facilities to carry out this work.
Citation: Birajdar AS, Meyyanathan SN (2022) Determination of Pseudoephedrine HCL and Levocetirizine HCL in Combination Dosage Form by High Pressure Liquid Chromatography. J Chromatogr Sep Tech. 13:465.
Received: 03-Jan-2022, Manuscript No. JCGST-22-15349; Editor assigned: 06-Jan-2022, Pre QC No. JCGST-22-15349 (PQ); Reviewed: 20-Jan-2022, QC No. JCGSR-22-15349; Revised: 24-Jan-2022, Manuscript No. JCGST-22-15349(R); Published: 31-Jan-2022 , DOI: 10.35248/2329-9096-22.10.629
Copyright: © 2022 Birajdar AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.