Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Review - (2019)Volume 9, Issue 1
Wilms Tumor is the most common primary renal tumor in childhood. Children with Wilms Tumor typically present with an asymptomatic abdominal mass, usually detected on a routine medical checkup or discovered coincidentally by parents. The initial differential diagnosis is with extrarenal abdominal masses; once a tumor of renal origin is established, distinguishing between Wilms Tumor and other primary renal neoplasms such as congenital mesoblastic nephroma, clear cell sarcoma, malignant rhabdoid tumor and renal cell carcinoma may not be easy. However, in many cases imaging findings in conjunction with the patient's clinical and epidemiological data, allow the diagnosis of Wilms Tumor. Wilms Tumor care offers one of the most striking examples of success of pediatric oncology. Over the last decades the European SIOP studies have been the key to developing standardized diagnostic procedures, improved risk stratification, and adjusted treatment recommendations for children with Wilms Tumor and this has resulted rate of overall survival is currently greater than 90%. As in previous SIOP trials and studies, the new protocol for the diagnosis and treatment of childhood renal tumors, the UMBRELLA SIOP–RTSG 2016, mandates preoperative chemotherapy without preceding mandatory histological assessment. Therefore, imaging studies are essential to obtain a presumptive diagnosis of WT, to provide disease staging information and to measure the tumor volume after neoadjuvant chemotherapy for the purposes of postoperative treatment stratification. This review describes role of imaging in the management of children with Wilms Tumor, according to the current recommendations of the UMBRELLA protocol.
Childhood kidney tumors; Wilms tumor; UMBRELLA protocol; Ultrasound; Computed tomography; Magnetic resonance imaging
Wilms Tumor (WT) is the most common childhood renal malignancy [1,2]. The prognosis is excellent and overall survival is currently greater than 90%. This achievement is due to the treatment advances over the last decades achieved through successive nationally and internationally based well organized coordinated clinical trials. Currently, the mission of the Renal Tumour Study Group of the International Society of Paediatric Oncology (SIOP–RTSG) is to minimize toxic therapies while maintaining excellent survival in all children diagnosed with any renal tumors [3]. On the basis of the experiences from SIOP–2001, the new protocol UMBRELLA SIOP-RTSG 2016 calls for management of patients with kidney neoplasm with initial chemotherapy without biopsy followed by surgery and adjuvant therapy with chemotherapy and/or radiation. Therefore, imaging studies are essential not only to determine the stage of the neoplasm, but above all to obtain a presumptive diagnosis of WT and minimize the risk of treating children with lesions other than WT with inappropriate chemothera®py. Moreover, after neoadjuvant chemotherapy, in addition to histological classification and neoplasm stage, the tumor volume measured using imaging is important for postoperative treatment stratification [1-3]. This review describes current role of imaging in the management of children with WT based on the recommendations of the UMBRELLA protocol and initially presents a brief overview of the epidemiological, etiological, histological and clinical features of the WT.
Childhood kidney tumors comprise about 5% of malignancies in children younger than 15 years [4]. In the pediatric age group WT, also known as nephroblastoma, accounts for about 90% of all tumors of the kidney [1,2,5]. WT and affects about 1/10,000 children worldwide before the age of 15 years and approximately 70 new cases are found each year in Italy [5-9]. In approximately 80% of cases WT occurs in children younger than 5 years of age and become less common as children grow older and it is seldom seen in neonates and adult [5,10,11]. WT is almost always solitary lesion, but about 12% of patients develop multifocal unilateral lesions and approximately 5%-7% of children can present with synchronous or metachronous bilateral disease [11,12]. WT is slightly more common in girls than in boys, especially in bilateral disease. Bilateral WT occurs at an earlier age than unilateral disease. The mean age at diagnosis is 45 months in unilateral cases and 32 months in bilateral cases A higher frequency of bilateral renal involvement is observed in patients with a predisposition to WT than in those without a genetic predisposition [5,6,11-13]. While vast majority of WTs are sporadic, in approximately 10% of cases WT is associated with genetic abnormalities, (aniridia, hemihypertrophy, genitourinary defects) or form part of several congenital syndromes, including WAGR syndrome (WT, aniridia, genitourinary abnormalities including ambiguous genitalia in males, and mental retardation), Denys-Drash syndrome (WT, male pseudohermaphroditism, and progressive glomerulonephritis) and Beckwith-Wiedemann Syndrome (BWS) (macroglossia, omphalocele, organomegaly, genitourinary anomalies, and increased risk for abdominal tumors) [6,8,10,11,13]. Only 2% of patients with WT have a relative with the disease. Children with congenital anomalies, syndromic conditions and a positive family history generally develop WT at an earlier age than in patients with sporadic disease [6,11,13-15]. Mutations of the Wilms Tumor 1 gene (WT1), located on the short arm of chromosome 11 (11p13) are found in majority of congenital anomalies and syndromes associated with WT and in only a minority of patients with sporadic or familial disease [5,6,8,11,13-16]. It is important to bear in mind that children with syndromes associated with WT1 mutations, are at increased risk of End-Stage Renal Disease (ESRD) [2,12]. Histologically, WT is a heterogeneous embryonal neoplasm composed of cells at different stages of differentiation that are normally seen in the developing kidney. Classical WT shows a triphasic histological pattern consisting of blastemal, epithelial and stromal elements in various proportions; monophasic variants with predominance of only one type of cell and biphasic variants with two main cellular elements, can also occur often [1,5,11,13,15- 19]. Blastema represents the least differentiated and most malignant component and consists of small round blue cells with overlapping nuclei, scanty cytoplasm and brisk mitotic activity. The epithelial component typically consists of embryonic tubular and glomerular-like structures while stromal pattern is mainly composed of spindle cells, which are mostly undifferentiated. Heterologous differentiation of epithelial or stromal elements, which includes mucinous or squamous epithelium, smooth or skeletal muscle, fat tissue, cartilage, bone and glial tissue, is present especially in tumors that have undergone preoperative chemotherapy [5,11,17-19]. An important histological feature of WT is anaplasia which may occur in any of the components of the tumor and is often seen in the blastemal elements. Anaplasia is found in approximately 5%-10% of WTs and the patients are usually older than those with non-anaplastic tumors [1,7,11,13,16-19]. Histologically, anaplasia is classified as focal and diffuse and is characterized by: large atypical tri/ multipolar mitotic figures; marked nuclear enlargement, with nuclear diameters at least three times those of adjacent cells; and hyperchromatic tumor cell nuclei. All three criteria for anaplasia have to be met in order to make the diagnosis [1,7,8,16-19]. Anaplasia is associated with TP53 mutations and it has a less favorable prognosis in the SIOP histological classification system. In particular, diffuse anaplasia is resistant to chemotherapy and carries a strongly elevated risk for relapse and fatal outcome in advanced disease stage [1,7,8,11,13,15-19]. A peculiar feature of WT is its association with Nephrogenic Rests (NRs) which are foci of persistent embryonic cells in the kidney after 36 weeks of gestation. NRs are recognized as pre-neoplastic proliferative processes associated with a high risk of developing WT [1,5,6,8,10,11,13,14,17-19]. These precursor lesions are identified in 30%-40% of kidneys which develop unilateral WT and in nearly all cases of bilateral WT; moreover, they are found in approximately 1% of infant kidneys at autopsy [1,5,8,10,11,14,16,17,19]. NRs found within a kidney resected for a WT increase the risk of WT in the contralateral kidney and, therefore, the patient requires close monitoring [19]. Anatomically, NRs are subdivided into two types: Intralobar Nephrogenic Rests (ILNRs) which are usually solitary and tend to be situated centrally within the kidney and Perilobar Nephrogenic Rests (PLNRs), which are confined to the periphery of the renal lobe and are often multiple [1,5,6,8,10,11,13,14,16-18]. Generally, NRs undergo gradual regression and only 1% of these lesions transform into WT; moreover, although PLNRs are more common than ILNRs, the risk of malignant transformation is much higher in the ILNRs [5,10,11,16,17]. Most NRs occur sporadically, but sometimes NRs present many of the same chromosomal defects as a WT and are associated with different syndromes and congenital anomalies: ILNRs are typically seen in children with sporadic aniridia and with WAGR and Denys-Drash syndromes, while PLNRs are usually found in the overgrowth conditions such as hemihypertrophy with or without BWS syndrome [1,6,8,11,13,14,17,19]. Nephroblastomatosis (NBL) is the term used to describe the presence of multiple or diffuse NRs [1,5,8,10,11,14,17,18]. In the Diffuse Hyperplastic Perilobar Nephroblastomatosis (DHPLN), the cortical surface of kidney is composed partially or entirely of proliferating nephroblastic tissue. The disease is often bilateral and associated with an increased risk of developing WT [1,11,17,18]. Clinically, the most common presentation of the WT is an asymptomatic abdominal mass often found by parents while bathing or clothing an affected child or by a physician on routine physical examination; in about 10% of cases mass can be discovered incidentally after trauma. Associated signs and symptoms such as malaise, pain, hypertension (presumably due to increased renin activity) and either microscopic or gross hematuria are found in about 20%-30% of the patients [5,8,10,11,13,14,17,19]. About 8% of patients may present clinical signs of acute abdomen with rapid abdominal enlargement, pain, anemia, hypotension and fever, suggestive of intraperitoneal hemorrhage due to the tumor rupture [5,11,13]. In about 11% of cases WT invades blood vessels in the form of tumor thrombus; in most of patients the tumor extends into renal vein, but extension of tumor thrombus into the Inferior Vena Cava (IVC) and, rarely, into the right atrium also can occurs [5,8,10,11,13,14]. Hematogenous metastases at diagnosis occur in 10% to 15% of children. The most common sites of metastasis are the lungs (85% of cases) and liver (20%), rarely bone. Local and distant Lymph Node (LN) involvement can also occur [5,10,11,13,14,19].
Imaging techniques and diagnostic findings
According to the UMBRELLA protocol, Ultrasound (US) is the mandatory first line imaging modality in children with a suspected abdominal mass. US is non-invasive and does not use Ionizing Radiation (IR). In children the small amount of tissue and fat allows application of a highresolution linear transducer, which offers excellent imaging capabilities; furthermore, US is applicable at the bedside and can be performed without the child having to be sedated [5,8,10,11,13,14]. US evaluates whether the mass originates from the kidney and whether it is solid or cystic and allows detailed examination of the contralateral kidney in search for a bilateral tumor, NRs or genitourinary malformations that may affect renal function [5,13]. In addition, this study is used to real-time evaluate relationship of the tumor with adjacent organs, provides initial disease staging information detecting the possible presence of lymph nodes metastasis, hepatic lesions or intraperitoneal implants and accurately assesses the presence or absence of abdominal or pelvic fluid (ascites or blood). At US, WT typically appears as a large, solid intrarenal mass with smooth and well-defined margins; it forms a pseudocapsule and shows heterogeneous echotexture: areas of necrosis and cysts appear hypoechoic and anechoic, while hyperechoic areas may represent haemorrhage and less frequently fat and calcifications (Figure 1). Because there is a risk that the tumor could rupture, the sonographer should carefully look outline of the tumor capsule and the presence of blood into the abdominal cavity. Pelvi-calyceal collecting system may be dilated as a result of the compressive effect of the mass [5,11,13,14]. WT often invades neighboring structures and during the ultrasound examination the movement of the mass can exclude invasion or adherence to adjacent organs of tumor [20]. Moreover, US is also the procedure of choice for examining the renal vein and IVC in search for intravenous tumor thrombus, both with 2D-ultrasound and Color-Doppler. Since US is an operatordependent technique and the restricted field-of-view may preclude complete assessment of the tumor's invasion of nearby areas, additional imaging studies for further characterization and accurate staging of WT are needed [5,11,13,14,20]. Magnetic Resonance Imaging (MRI) of the abdomen is the first-choice complementary imaging procedure to US, as the UMBRELLA protocol encourages use of techniques which do not employ IR to generate images. In effect, it is important to consider the ionizing hazards of CT in children with WT who will need many imaging studies. MRI also is the imaging method with the best soft-tissue contrast. It accurately depicts the primary tumor, its renal origin and the relation of the tumor to other organs and detects intra-abdominal metastatic disease [5,11,20]. At MR imaging, the tumor is typically heterogeneous, lobulated and is generally hypointense on T1-weighted images and hyperintense on T2-weighted images; after intravenous administration of gadolinium contrast material WT displays enhancement to a lesser degree than the normal renal cortex, but the heterogeneity of the tumor increases due to haemorrhage, necrosis and cysts. Intratumoral hemorrhage is typically evident on noncontrast MR imaging as an area of T1 high signal intensity. In a minority of cases, WT may be predominantly cystic, an entity known as cystic partially differentiated nephroblastoma, that may be difficult to distinguish from other multicystic renal neoplasms, both benign and malignant, although the presence of solid nodules in the septa can help narrow the differential diagnosis. WT may distort the architecture of the kidney and MRI can demonstrate the ‘claw sign’ of normal renal tissue around the tumor. Moreover, MRI accurately evaluates tumor thrombus extension into the renal vein, IVC, up to the level of the hepatic veins or even into the right atrium [8,10,11,14,20]. MRI is especially beneficial in children with suspected bilateral disease and with small lesions and it is recommended as best practice to differentiate NRs from WT. At MR imaging, NRs are hypointense relative to normal renal cortex on T1-weighted images and iso- to slightly hyperintense on T2-weighted images. Moreover, lesions are often best seen on T1-weighted images after gadolinium administration, because become sharply hypointense in comparison with the intensely enhancing renal parenchyma [8,10,11,14,20]. Unlike WT, the feature of NRs at diagnosis being their diffuse homogeneity both before and after contrast agent administration [8,10,11,14,20,21]. Techniques such as Diffusion Weighted Imaging (DWI) with Apparent Diffusion Coefficient (ADC) mapping can be added to the classical sequences (sequences before and after contrast agent administration and with T1, T2 and STIR sequences) to give biological information about the composition of tissues and their physical properties. DWI provides images based on the molecular motion of the water inside the tissue [5,20,22,23]. Most malignant lesions show restricted diffusion due to the dense cellular packing of solid tumors and increased cell membranes per unit volume and this involves high signal intensity on DWI. The degree of water molecules diffusion can be evaluated quantitatively by the ADC, which is calculated for each pixel of the image and is displayed as a parametric map. The ADC value is inversely proportional to cellular density: areas of restricted diffusion represent high cellular areas with low ADC values and hypointense signal in ADC maps. WT is characterized by high cellularity, small, round, relatively undifferentiated cells, determining restriction on DW images represented as high signal while ADC map reveals the low signal intensity of the tumor [21,24] (Figures 2 and 3). Anyway, is not always possible to differentiate between malignant and benign lesions on the basis of ADC values alone. The region of interest can include necrotic or cystic areas which render ADC measurement ineffective and difference in ADC values between benign and malignant lesions may not be statistically significant [20-24]. However, DWI can help differentiate between viable and necrotic areas within tumors at diagnosis and after preoperative chemotherapy and can be useful in guiding biopsies. In fact, identification of a remarkable proportion of relatively low ADC values can guide the proper selection of the biopsy site in order to detect malignant blastema component and determine the most appropriate treatment for patient [20,23,25,26]. Finally, DWI cannot distinguish with certainty WT from NBL/NRs, based on mean ADC values, but allows better visualization of small NBL foci and can also detect additional NBL lesions not seen on the classical MRI sequences both at presentation and after neoadjuvant chemotherapy [3,24]. MRI can be difficult to perform in children due to their inability to stay still during the examination and sedation may be necessary not without risk of adverse events. According to the UMBRELLA protocol, Computed Tomography (CT) of the abdomen can be performed only if MRI is not available. CT is readily available and so fast that most children do not need to be sedated, but IR exposure remains its major disadvantage [5,8,11,20]. Therefore, radiation dose should be kept As Low As is Reasonably Achievable (ALARA) and the CT technique will be adjusted to the size of children to limit the delivered dose with no notable impact on the quality of the exam. Administration of intravenous iodinated contrast is mandatory and usually only one single portal venous phase is sufficient for diagnosis [11,20,27]. On CT, WT appears as a heterogeneous soft-tissue density mass that shows less enhancement than adjacent normal renal parenchyma. Enhancement is also patchy and allows better delineation of the relationship of the tumor with kidney. WT usually contains hypodense areas due to necrosis, old hemorrhage and cysts, but fat and calcifications may also be found. CT can also show the ‘claw sign’ and reconstructed images in sagittal and coronal planes are useful for assessing tumor extent. The other CT findings may include infiltration and distortion of the calyceal system, vascular invasion and LN metastases [8,10,11,14,20] (Figures 4 and 5). CT provides accurate local staging of disease and detects the presence of NRs within kidney (Figure 6). However, MRI is considered the most accurate imaging modality for assessing venous thrombus and it is more sensitive to detect small contralateral lesions [3,8,11,14,24,28]. According to the UMBRELLA protocol, chest CT is a mandatory diagnostic procedure to assess lung metastasis as it is more sensitive than conventional radiography for detecting very small lung nodules (Figure 7). Intravenous contrast is not mandatory, but may be used if it is combined with an abdominal CT scan instead of abdominal MRI. Chest X-ray with Anteroposterior (AP) or Posteroanterior (PA) view will be performed at diagnosis as a mandatory baseline procedure and also will serve as test for the control of an implanted central catheter [5,8,11,20]. The increasing use of chest CT as routine imaging technique for staging has resulted in the detection of small pulmonary lesions not visible on radiograph, known as CT-only nodules. This carries from one side a much higher burden of IR exposure compared to chest X-ray and from the other the risk of overdiagnosing metastasis as often the CT‑only nodules that occur in children are benign [5,11,20]. However, results from the Children's Oncology Group (COG) National Wilms Tumor Study Group (NWTS)-4 and NWTS-5 trials showed that patients with CT only nodules who were treated with therapy for metastatic disease had superior Event-Free Survival (EFS) to those treated with therapy for localized disease, but overall survival was similar in both groups. It follows that, the majority of these lesions represent metastases and currently in the UMBRELLA protocol CT‑only nodules are treated as metastases if they have a transverse diameter of at least 3 mm [2,11,13,29]. Finally, given that abdominal CT is the most common imaging tests performed for the investigation of acute abdominal pathology, it is important that it demonstrates the presence of tumor rupture, which is a major risk factor for abdominal recurrence [5,7,12,20]. In the study by Le Rouzic et al., the CT demonstrates good sensitivity for detecting acute hemorrhage, identified on non-contrast image as an area of high density. Less specific imaging findings include perirenal hemorrhage that can be present without visualization of the rupture of tumor, retroperitoneal fluid and intra-abdominal peritoneal effusion [30]. According to the UMBRELLA protocol, tumor rupture at diagnosis on imaging studies upstages disease only if it can be detected by pathological examination of the nephrectomy specimen (Figure 8). If rupture is confirmed, children will receive or not abdominal Radiotherapy (RT) along with chemotherapy depending on risk stratification after surgery [1,2]. However, preoperative chemotherapy determines development of a peritumoral capsule, which can mask tumor rupture on histological analysis with the resulting risk of relapse as patients can be treated without considering an obvious initial rupture later refuted on histological examination. Moreover, Le Rouzic et al. reported that postchemotherapy CT evaluation of tumor rupture lacked sensitivity and specificity because none of the patients with histologically proven rupture who presented radiological signs at diagnosis presented persistent radiological criteria on preoperative CT evaluation. On the other hand, CT may overestimate the tumor ruptures, leading to overtreatment in some patients, though tumor rupture is not detected by pathological examination, with the resulting risk of longterm adverse effects of RT and chemotherapy. Currently, there are no recommendations in case of discrepancy between radiological and histological signs of rupture at diagnosis and after neoadjuvant chemotherapy and physicians may have difficulty making the therapeutic decisions [30].
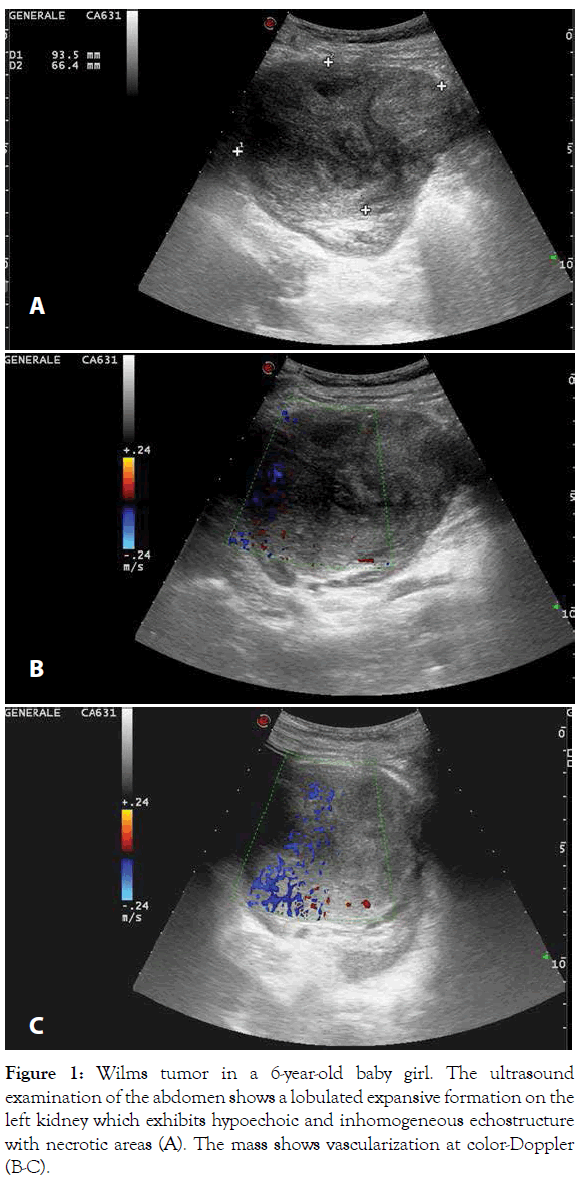
Figure 1: Wilms tumor in a 6-year-old baby girl. The ultrasound examination of the abdomen shows a lobulated expansive formation on the left kidney which exhibits hypoechoic and inhomogeneous echostructure with necrotic areas (A). The mass shows vascularization at color-Doppler (B-C).
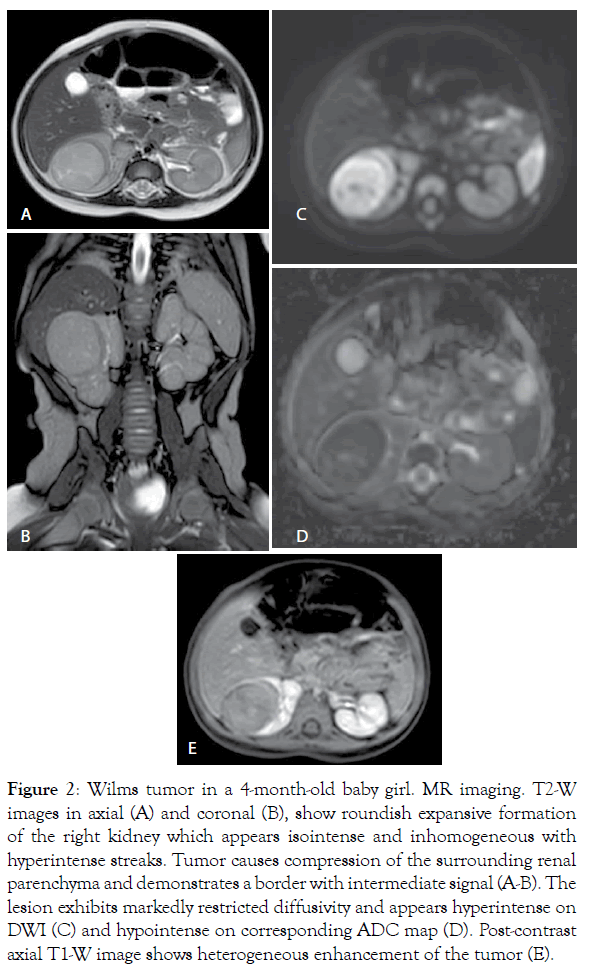
Figure 2: Wilms tumor in a 4-month-old baby girl. MR imaging. T2-W images in axial (A) and coronal (B), show roundish expansive formation of the right kidney which appears isointense and inhomogeneous with hyperintense streaks. Tumor causes compression of the surrounding renal parenchyma and demonstrates a border with intermediate signal (A-B). The lesion exhibits markedly restricted diffusivity and appears hyperintense on DWI (C) and hypointense on corresponding ADC map (D). Post-contrast axial T1-W image shows heterogeneous enhancement of the tumor (E).
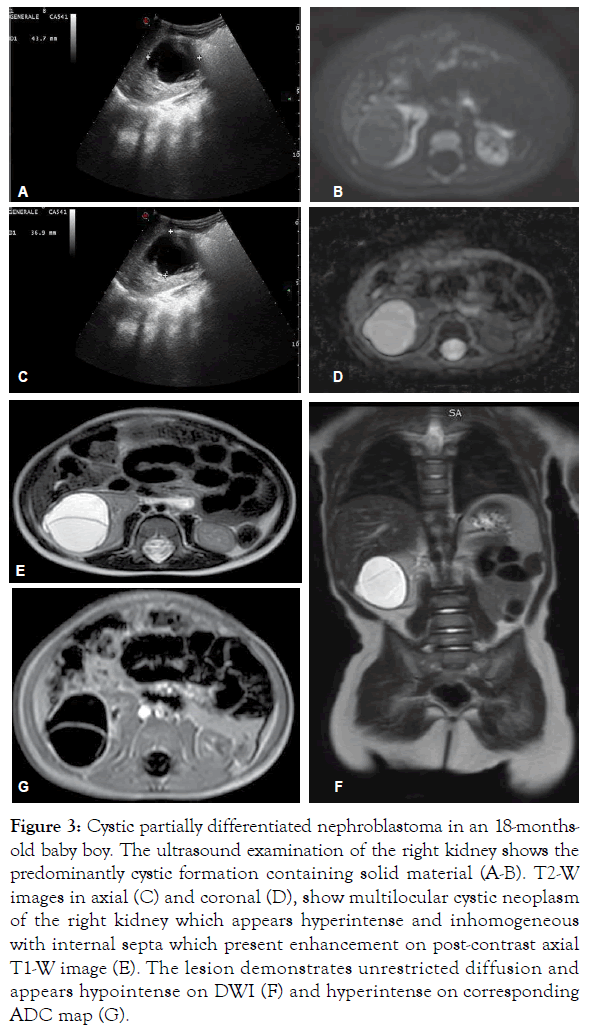
Figure 3: Cystic partially differentiated nephroblastoma in an 18-monthsold baby boy. The ultrasound examination of the right kidney shows the predominantly cystic formation containing solid material (A-B). T2-W images in axial (C) and coronal (D), show multilocular cystic neoplasm of the right kidney which appears hyperintense and inhomogeneous with internal septa which present enhancement on post-contrast axial T1-W image (E). The lesion demonstrates unrestricted diffusion and appears hypointense on DWI (F) and hyperintense on corresponding ADC map (G).
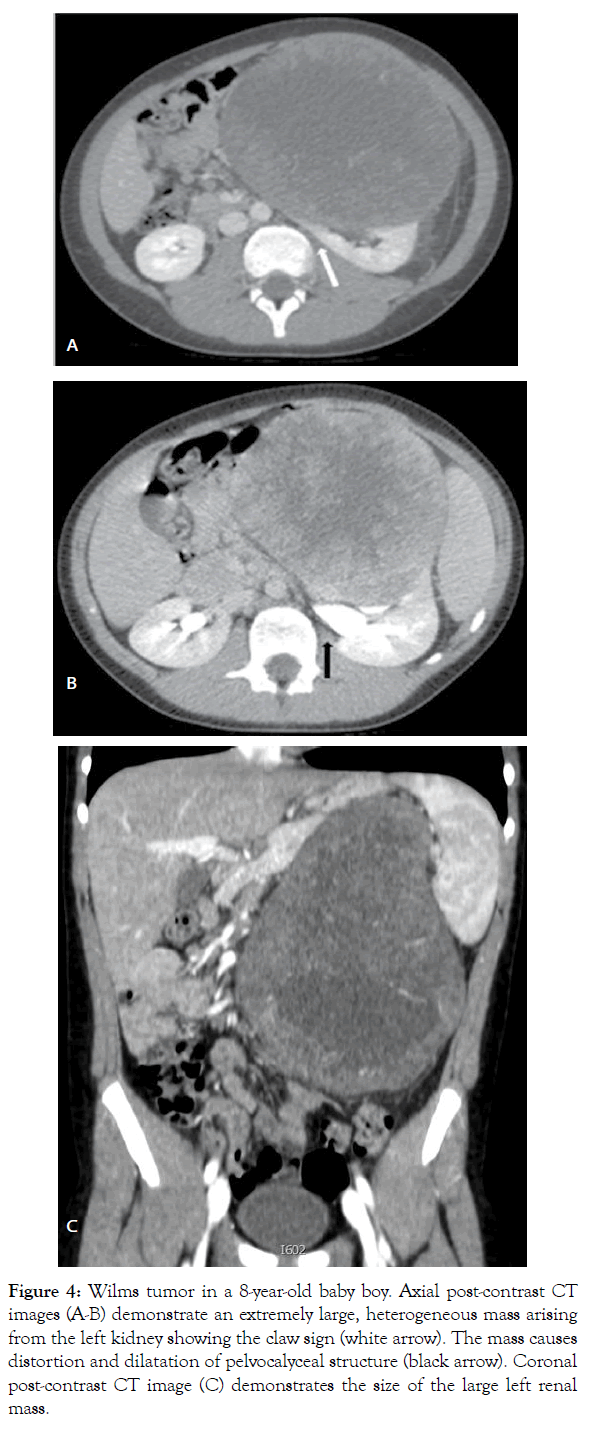
Figure 4: Wilms tumor in a 8-year-old baby boy. Axial post-contrast CT images (A-B) demonstrate an extremely large, heterogeneous mass arising from the left kidney showing the claw sign (white arrow). The mass causes distortion and dilatation of pelvocalyceal structure (black arrow). Coronal post-contrast CT image (C) demonstrates the size of the large left renal mass.
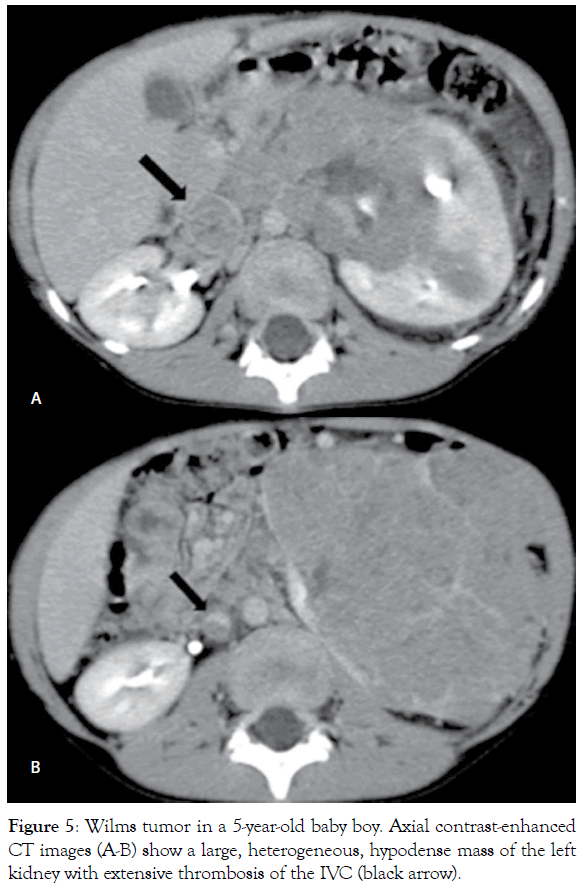
Figure 5: Wilms tumor in a 5-year-old baby boy. Axial contrast-enhanced CT images (A-B) show a large, heterogeneous, hypodense mass of the left kidney with extensive thrombosis of the IVC (black arrow).
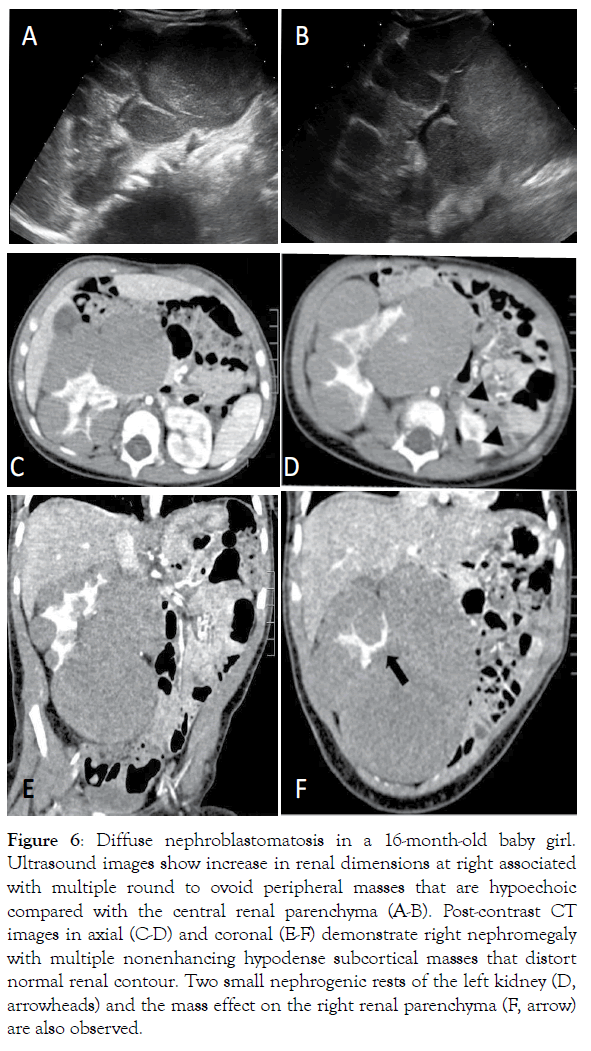
Figure 6: Diffuse nephroblastomatosis in a 16-month-old baby girl. Ultrasound images show increase in renal dimensions at right associated with multiple round to ovoid peripheral masses that are hypoechoic compared with the central renal parenchyma (A-B). Post-contrast CT images in axial (C-D) and coronal (E-F) demonstrate right nephromegaly with multiple nonenhancing hypodense subcortical masses that distort normal renal contour. Two small nephrogenic rests of the left kidney (D, arrowheads) and the mass effect on the right renal parenchyma (F, arrow) are also observed.
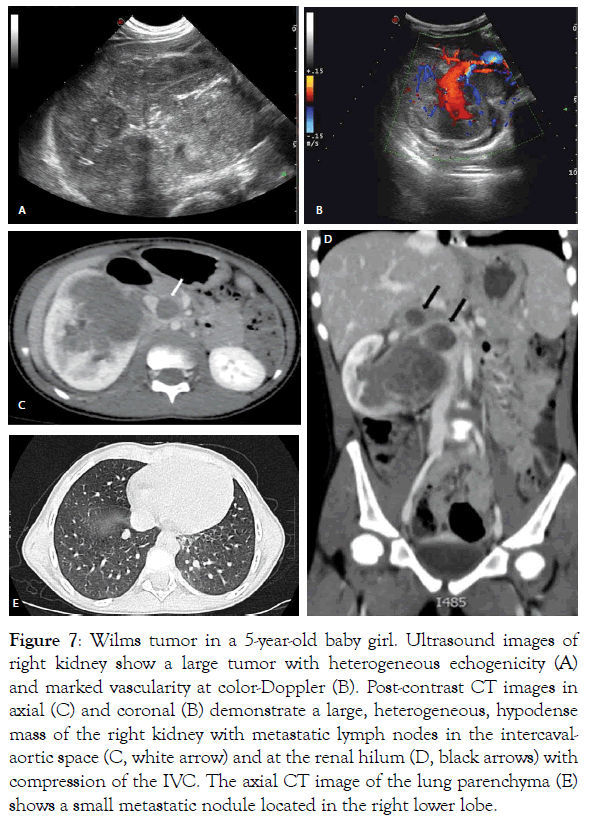
Figure 7: Wilms tumor in a 5-year-old baby girl. Ultrasound images of right kidney show a large tumor with heterogeneous echogenicity (A) and marked vascularity at color-Doppler (B). Post-contrast CT images in axial (C) and coronal (B) demonstrate a large, heterogeneous, hypodense mass of the right kidney with metastatic lymph nodes in the intercavalaortic space (C, white arrow) and at the renal hilum (D, black arrows) with compression of the IVC. The axial CT image of the lung parenchyma (E) shows a small metastatic nodule located in the right lower lobe.
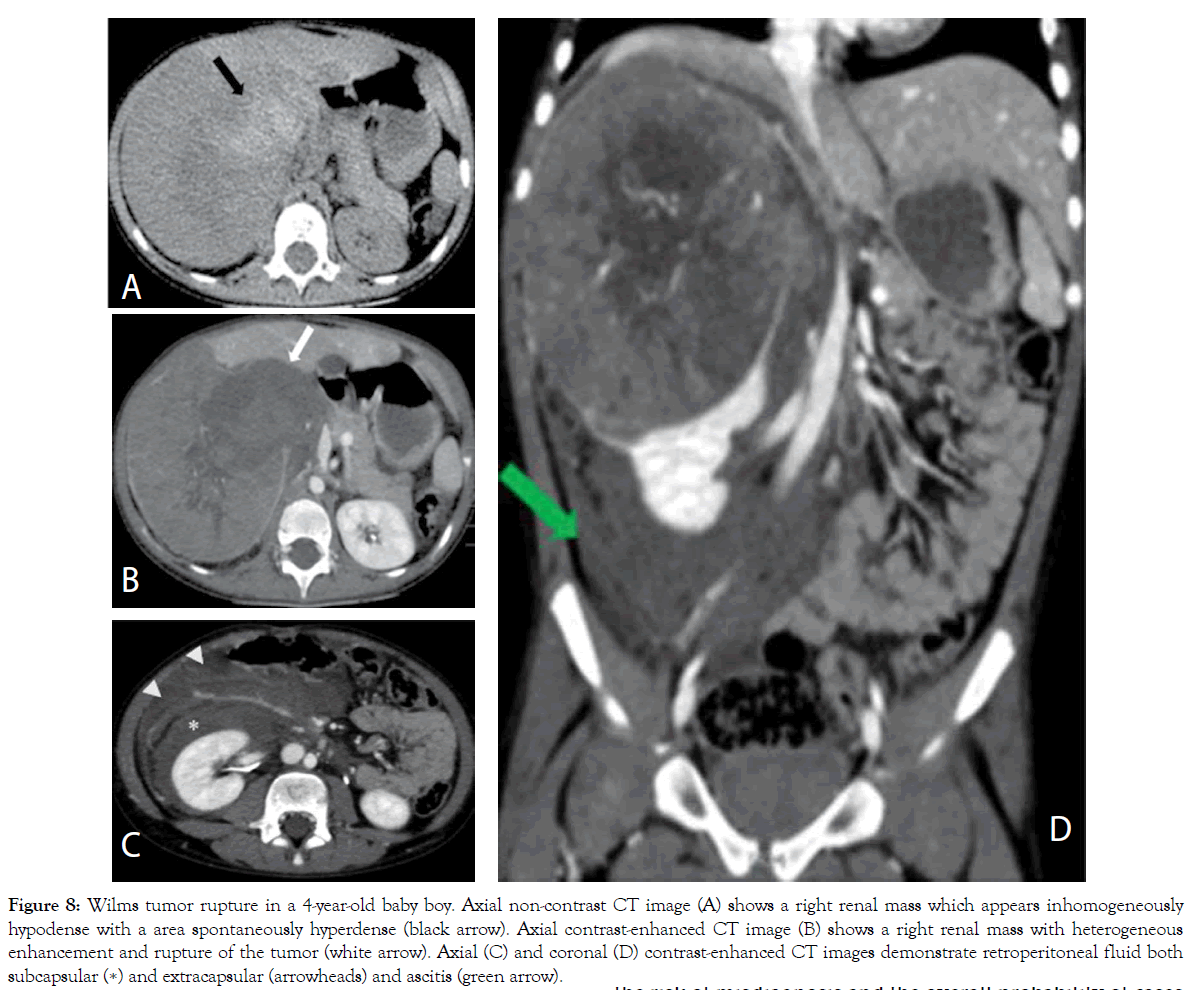
Figure 8: Wilms tumor rupture in a 4-year-old baby boy. Axial non-contrast CT image (A) shows a right renal mass which appears inhomogeneously hypodense with a area spontaneously hyperdense (black arrow). Axial contrast-enhanced CT image (B) shows a right renal mass with heterogeneous enhancement and rupture of the tumor (white arrow). Axial (C) and coronal (D) contrast-enhanced CT images demonstrate retroperitoneal fluid both subcapsular (∗) and extracapsular (arrowheads) and ascitis (green arrow).
Management strategy and differential diagnosis
In children with renal tumors, the UMBRELLA protocol mandates preoperative chemotherapy followed by surgery and the diagnosis will be confirmed on pathological examination of the pretreated nephrectomy specimens. WT is often large and fragile and consequently is prone to rupture during surgery. Neoadjuvant chemotherapy (vincristine and actinomycin-D in localized and with the addition of doxorubicin in metastatic disease) reduces tumor size facilitating subsequent surgery and decreasing the risk of intraoperative tumor rupture [1- 3,5,7,11,13,14,17,20,25,26,28,29] (Figure 9). Also, preoperative therapy facilitates surgical resection by decreasing the extent of the tumor thrombus and so reduces risks of massive haemorrhage or pulmonary embolus during surgery [20]. According to the SIOP–RTSG, diagnostic biopsy of renal tumors prior to neoadjuvant chemotherapy is not mandatory as WT is the most common form of kidney cancer among children and this approach can avoid a delay in treatment and the rare but possible complications that might take place after a biopsy procedure. Biopsy is also contraindicated in patients with bilateral or multifocal tumors, as it is very difficult or impossible to distinguish between WT and NRs in limited needle biopsy material [1,2,5,8,11,12,17,18,20,21,24]. Since the UMBRELLA protocol does not require histological confirmation before neoadjuvant chemotherapy, clinical manifestations, laboratory tests and imaging findings are essential to obtain a presumptive diagnosis of WT to guide therapeutic decisions [1-3,11,13,20]. The initial differential diagnosis is with extrarenal abdominal masses; once a tumor of renal origin is established, conditions to be considered in the differential diagnosis of WT include less common kidney tumors such as Congenital Mesoblastic Nephroma (CMN), Clear Cell Sarcoma of the Kidney (CCSK), Malignant Rhabdoid Tumor of the Kidney (MRTK) and Renal Cell Carcinoma (RCC), which are also called non-Wilms Tumors (non-WT). Non-WT are usually indistinguishable from WT on imaging studies [1,2,8,10,11,13,14,20]. CMN is the predominant renal neoplasm in the first 3 months of life and is uncommon after 6 months. CMN is histologically divided into classic and cellular subtypes [8,11,13,14,20,31]. At imaging, classic CMN appears as a homogeneous solid mass, while cellular form generally is heterogeneous with areas of necrosis, hemorrhage and cystic degeneration; moreover, cellular CMN tends to be larger and present later in infancy and can exhibit aggressive behavior including encasement of vessels and invasion of adjacent organs [11,14,20,31]. CMN is usually a benign tumor, but sometimes local recurrences occur and distant metastases have been observed in cellular CMN, with the main site of metastasis the lung [8,11,14,20,31]. CCSK is known for being difficult to diagnose because has a mean age of presentation overlapping with WT and it can be indistinguishable from WT on imaging. Unlike WT, CCSK does not invade vessels and has a unique propensity to metastasize to bone and brain [8,10,11,13,14,20]. According to the UMBRELLA protocol, due to the predilection of CCSK to metastasize to bone, technetium bone scan or whole-body MRI or FDGPET should be performed once the diagnosis is established. Brain MRI is also recommended in children with histologically confirmed CCSK [11,20]. MRTK typically occurs in children younger than 2 years of age (mean age 11 months) and it is extremely rare after 5 years of age [5,8,11,14]. On CT, MRTK may present as a large, heterogeneous soft-tissue mass and may be indistinguishable from WT. In fact, more specific imaging characteristics such as subcapsular fluid collections, tumor lobules outlined by linear calcification or separated by areas of necrosis or haemorrhage and the voluminous hilar or retroperitoneal lymphadenopathies, are not always present Moreover, given the rarity of rhabdoid tumor, it is hard to believe that renal mass is not WT [8,11,14,20]. The association of MRTK with synchronous or metachronous primary intracranial masses or brain metastases, particularly in the posterior fossa, has been recognized as a distinctive feature. Therefore, brain MRI have to be performed in children with histologically proven MRTK. Moreover, like WT, MRTK spreads to the lungs and liver [8,10,11,13,14,20]. Finally, RCC is more common than WT in adolescents and the average age at diagnosis is 10–11 years [8,11,14,32]. Specific imaging characteristics are few or absent. RCC appears smaller than WT, becomes progressively larger and can invade neighbouring tissues destroying normal renal architecture; compared to WT, RCC more often presents bilaterally and calcifications are more frequently seen. Regional lymphadenopathies and vascular invasion are commonly observed, but are also present in WT. Both WT and RCC tend to metastasize to the lung and liver, but RCC is more likely to spread to the bone. According to the UMBRELLA protocol, technetium bone scan or wholebody MRI or FDG-PET should be performed in children with histologically confirmed RCC [8,10,14,20,32]. The SIOP strategy that does not mandate a pretherapy biopsy, carries the risk of misdiagnosis and the overall probability of cases clinically and radiologically diagnosed as WT, that prove to be other tumors on histopathological examination, is about 10% [11,28,33,34]. In the study by Vujanic et al. in the 12% of patients that were clinically and radiologically consistent with WT, the biopsy revealed tumors other than WT and this rate was found to be higher in the study by Kurian et al., where 18.6% of all pediatric renal tumors were non- Wilms [34,35]. The SIOP–RTSG accounts for the risk of misdiagnosis of WT by recommending direct surgery instead of preoperative chemotherapy for children aged under 6 months due to the high prevalence of CMN in this age group and in the completely cystic tumors because needle sampling does not provide sufficient material for an accurate histological diagnosis [1,2,5,33]. Moreover, to reduce the chances of a non-Wilms histology, Percutaneous Cutting Needle Biopsy (PCNB), before neoadjuvant chemotherapy should be considered in the cases of: 1) Unusual clinical presentation: age>10 years, urinary infection, septicaemia, psoas inflammation/infiltration; 2) Unusual imaging findings for WT: voluminous lymphadenopathies, numerous calcifications, renal parenchyma not visible, almost totally extra-renal process, pulmonary metastasis<age of 2 years, extrahepatic and extrapulmonary metastases; 3) Biological finding: hypercalcaemia [1,2,20,33]. Age at diagnosis is an important clinical criterion for recommending biopsy because in individuals over 10 years of age a renal mass is more likely to be RCC than WT [1,8,11,14,20,32,33]. Urinary infection, septicaemia and psoas inflammation/infiltration, are part of the SIOP clinical criteria for the indication of biopsy in order to rule out renal inflammatory pseudotumors that may present as unilateral solitary renal mass on imaging and may be misdiagnosed as renal tumors [20,33]. In particular, Xanthogranulomatous Pyelonephritis (XGP) is the most common renal inflammatory lesion mimicking a WT because in children the disease is focal, localized and without calculi [33,36]. Among SIOP radiological criteria, the presence of voluminous hilar or retroperitoneal lymphadenopathies depicted by imaging is an unusual imaging finding requiring biopsy in order to rule out MRTK and RCC [8,11,14,20,32,33]. Other imaging features abnormal enough to require biopsy are numerous calcifications to exclude Neuroblastoma (NB), RCC and MRTK and the renal parenchyma not visible and almost totally extrarenal process to distinguish WT from NB with renal involvement [8,10,11,14,20,32,33]. Like WT, NB develops in the retroperitoneum and occurs mostly in children under the age of 5 years. Both WT and NB can present as large masses completely filling the retroperitoneal spaces and it is often difficult to make a clear distinction between the two types of tumors [11,14,20]. Current multiplanar imaging techniques are very useful to distinguish a renal mass from an extrarenal mass. At CT or MR imaging, renal origin is suggested by the “claw sign” of renal parenchyma surrounding the tumor, whereas NB displaces the ipsilateral kidney. In situations where the imaging demonstrates intratumoral calcifications, encasement of vessels and/or tumor crossing the midline behind the aorta, extension through neural foramina into the spinal canal and/or skeletal metastases, the diagnosis of NB is more common [11,14,20,33]. Moreover, elevations of the urine levels of the catecholamines supports diagnosis in more than 90% of cases Therefore, in all children with retroperitoneal tumors the assessment of urinary catecholamines is performed. Only in case of serious doubt of WT based on clinical presentation and imaging, PCNB and MIBG scintigraphy are recommended [20,33,37]. The presence of pulmonary metastases in children younger than 2 years of age, is an another unusual imaging finding requiring biopsy because there is strong suspicion that these children are affected by MRTK. Indeed, patients younger than two years with stage 4 WT, are an absolute rarity [8,5,11,14,20,33,38]. Moreover, extrapulmonary and extrahepatic metastases are uncommon imaging features in children with WT and biopsy should be performed in order to detect MRTK and CCSK possibly associated with brain or bone metastases and to identify RCC when skeletal metastases are found [8,10,11,13,14,20,32]. Finally, hypercalcemia due to secretion of parathormone by the tumor cells, is part of the SIOP biopsy criteria. Hypercalcemia occurs mainly in young children with MRTK and CMN and is uncommon in WT [8,11,14,33]. While children under the age of 6 months with a strong suspicion of CMN are subjected to upfront surgery, those older than 6 months can be affected by cellular CMN that is indistinguishable from WT at imaging. Therefore, hypercalcemia may serve as tumor marker and as criterion for the indication of biopsy [1,2,5,11,14,20,31,33]. The application of the SIOP criteria for the indication of biopsy has been shown to be effective.
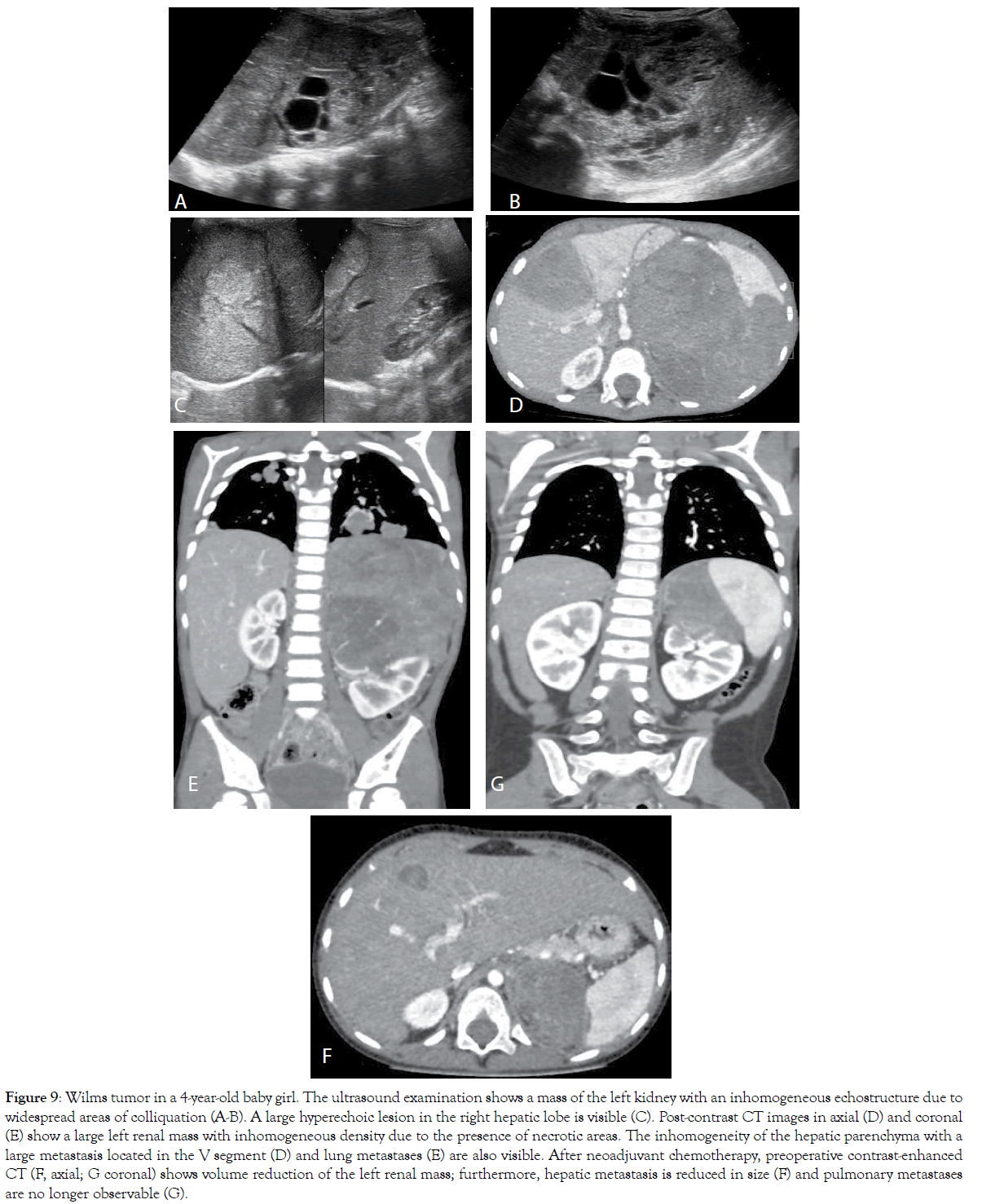
Figure 9: Wilms tumor in a 4-year-old baby girl. The ultrasound examination shows a mass of the left kidney with an inhomogeneous echostructure due to widespread areas of colliquation (A-B). A large hyperechoic lesion in the right hepatic lobe is visible (C). Post-contrast CT images in axial (D) and coronal (E) show a large left renal mass with inhomogeneous density due to the presence of necrotic areas. The inhomogeneity of the hepatic parenchyma with a large metastasis located in the V segment (D) and lung metastases (E) are also visible. After neoadjuvant chemotherapy, preoperative contrast-enhanced CT (F, axial; G coronal) shows volume reduction of the left renal mass; furthermore, hepatic metastasis is reduced in size (F) and pulmonary metastases are no longer observable (G).
In fact, in the recent study by de la Monneraye et al., the risk of misdiagnosis related to the SIOP strategy appears limited because only 3% of patients treated by presumptive chemotherapy finally have a non-WT [33]. When histological examination is necessary for diagnosis, PCNB is performed under general anaesthesia using large needles (16-18 Gauge) to guarantee sufficient tissue for pathologic differentiation and with the guidance of ultrasound or CT to make sure to sample solid and viable tumor and avoid sampling of necrotic or cystic areas [33-34]. Co-axial technique is mandatory because lowers the risk of local recurrence along the core-needle biopsy tract and retroperitoneal access carries little risk of disease dissemination, avoiding the peritoneal cavity [33,35]. Important complications from biopsy are possible, although rare. In the study by Vujanic et al. in 1.6% of cases PCNB led to relevant complications such as intratumoral bleeding, tumor rupture and needle track recurrence [34]. In the study by Kurian et al. biopsy site recurrence was seen in 1 child with bilateral WT, instead, Monneraye et al. have not found cases of lumbar wall needle tract relapse [33,35]. Finally, it is important to stress that PCNB does not upstage the tumor, but anaplastic histology can be focal and missed at biopsy due to sampling error [1,11,33].
Neoadjuvant therapy reduces the size of the renal tumor for most patients and imaging studies before surgery are designed to evaluate the extent of the renal mass, its location within the kidney and its relationship to the adjacent organs. Involvement of regional LNs, involvement of the other kidney, spread to the peritoneum, thrombus in renal vein, IVC or right atrium and metastatic disease (liver and lung) must also be identified. According to the UMBRELLA protocol, abdominal MRI (or CT) and chest CT (only in presence of lung metastases at diagnosis) must be repeated before abdominal surgery [8,10,11,13,14,20]. Multiplanar acquisitions with MRI or CT are extremely helpful in depicting the surgical possibilities and margins, especially for children with synchronous bilateral WT. Patients with bilateral disease have an increased risk of ESRD due to underlying germline genetic aberrations and due to treatment-related loss of kidney function. Children receive preoperative chemotherapy with vincristine and actinomycin D and subsequent reduction of lesions size and volume allows Nephron-Sparing Surgery (NSS), whose objective is to preserve maximum of healthy parenchyma in both kidneys [1,2,5,11-13,20,21,24,26]. Preoperative imaging plays a crucial role in determining the resectability of lesions, but occasionally it can't detect viable renal parenchyma due to the volume effect of large masses [12,20,21,24,26,39]. NSS is also considered alternative to total nephrectomy in the treatment of unilateral WT in children with tumor involving a solitary or horseshoe kidney, in the cases of genitourinary abnormalities of the other kidney endangering the renal function and in children with syndromes which predispose to metachronous development of WT in the contralateral kidney [5,11,12,20]. Cases of simple unilateral disease may also benefit from NSS and some indications for this procedure can be detected by the radiologist. According to the UMBRELLA protocol, renal scintigraphy with Dimercaptosuccinic Acid (DMSA) should be considered prior to surgery and NSS is acceptable in cases of unilateral non-syndromic WT provided the following criteria are met: 1. Tumor restricted to one pole of kidney or peripheral at mid-kidney; 2. Volume<300 mL at diagnosis (the risk of positive LNs is about 2% in small tumors, but following NSS, positive LNs on histological examination can indicate radiotherapy and not necessarily a completion nephrectomy); 3. No preoperative rupture (the imaging criteria for rupture: retroperitoneal peritumoral effusion/hemorrhage, peritoneal hemorrhage or nodules, spontaneously or by open biopsy and no intraoperative rupture); 4. No intraluminal tumor on preoperative imaging in renal pelvis; 5. No invasion of surrounding organs; 6. No thrombus in the renal vein or IVC; 7. No multifocal tumor; 8. Excision can be performed with oncological safe margin; 9. Kidney remnant is expected to show remaining function; 10. At least 66% of renal tissue should be spared after the tumor resection with a margin of healthy tissue, to give any worthwhile protection against hyperperfusion. If this is in doubt pre-operative DMSA may be able to define expected post-operative function. In case of visceral metastasis, NSS should not be systematically ruled out but considered carefully. Resection must be performed with margins of healthy renal tissue. Intraoperative US can be useful in defining the tumor margins more accurately. In cases of microscopically incomplete resection and in presence of unfavourable subtypes of renal tumors, complete nephrectomy seems necessary. Following NSS, the remaining kidney should be carefully examined and Doppler US is performed 2 days postoperatively [1,2,5,11,20]. The staging system by SIOP is performed in consideration of the results of imaging studies, as well as histopathological findings after surgery (Table 1). Neoadjuvant chemotherapy can downstage disease prior to surgery and the shift towards a more advantageous stage results in reduced postoperative treatment intensity [1- 3,5,8,20,26,33]. Results of a randomized trial (UKW3) by the UK Children’s Cancer Study Group, show that in children receiving preoperative chemotherapy with vincristine and actinomycin D for non-metastatic WT the use of radiotherapy or doxorubicin could be reduced by 20% compared to patients treated with direct nephrectomy, with no significant difference in survival. Therefore, these children can be spared from the late-effects of doxorubicin or radiotherapy [2,5,40]. UMBRELLA protocol uses postsurgical pathological staging, but also histological risk group assigned by assessment of the tumor's response to neoadjuvant chemotherapy to plan postoperative treatment [1-3,5,7,17,18,21,26]. Histological assignment to the different risk groups is based on quantification of Chemotherapy- Induced Changes (CIC). CIC include necrosis, haemorrhage and fibrosis of varying degree and areas with foamy macrophages and/or hemosiderin-laden macrophages. Other CIC include maturation of blastemal, epithelial and stromal components. Highly proliferative blastemal component more readily responds to chemotherapy, while mature epithelial and stromal components are often less sensitive to chemotherapy and a significant reduction in the size of these tumors may not be obvious after preoperative chemotherapy [1,7,18,20,25,26]. Histologic changes after chemotherapy define three major types of WT: low-risk, high-risk and intermediate-risk tumors (Table 2). The lowrisk group includes WTs that respond to preoperative chemotherapy so well that no viable tumor is found at nephrectomy (completely necrotic-type). Tumors in other risk groups are subclassified on the basis of the percentages of CIC and viable tumor components. The criteria for subclassifying pre-treated WTs are as follows: completely necrotic type shows no viable tumor elements. If more than 66% (two-thirds) of the tumor is non-viable it is regarded as regressive type, irrespectively of the presence of remaining viable tumor components (except anaplasia). If viable tumor comprises more than one-third of the tumor mass, subtyping depends on the percentage of viable components: in mixed type no component comprises more than 66% of tumor; in epithelial (or stromal) type more than 66% of the tumor is composed of epithelial (or stromal) elements and in addition only up to 10% of blastema is allowed (if the finding is more, the tumor is subclassified as mixed type) (Table 3) [1,7,18,26]. A subgroup of patients showing persistence of chemotherapy-resistant blastemal cells after preoperative chemotherapy remains at high risk of relapse and requires intensive post-surgery chemotherapy [1- 3,5,7,17,18,21,25,26]. In the UMBRELLA study the volume of blastema in tumor is not a criterion for treatment stratification. The results of the SIOP 2001 WT trial showed that patients with localized WT and a residual blastemal volume of more than 20 mL did significantly worse than those with a smaller blastemal volume. This criterion is being prospectively assessed in the UMBRELLA study in order to validate the prognostic significance of blastemal volume with the ultimate goal to improve treatment stratification [1-3,5,26,41]. DWI MRI is a functional imaging tool that can help evaluate response to neoadjuvant treatment. In fact, tumors responsive to chemotherapy are reduced in size and show an increase in ADC values, probably due to tumor necrosis, reduced cellularity and concomitant tissue edema, instead, non-responding lesions have persistent low ADC values. Moreover, DWI MRI can help predict histological risk group by providing an estimate of the cell density of the tumor tissue and thus information on apoptotic effects of chemotherapy [3,20,21,23-26]. In recent studies, DWI MRI has been effective in stratification of histological subtypes of WT by using ADC values of residual viable parts of tumor and showed lower ADC values in the high-risk blastemal type compared with subtypes of the intermediate risk (stromal, regressive and mixed). Instead, no significant difference in ADC was found between blastemal type WT and intermediate risk epithelial type, because both demonstrated relatively low ADC values. Since that blastema is the most responsive tumor component to chemotherapy, the combination of evident tumor reduction or necrosis with relative low ADC values in viable tumor components could be strongly indicative of chemo-resistant blastema [21,24- 26]. On the other hand, the linear relationship between stromal histopathology and ADC values is very important as following chemotherapy, stromal predominant WTs often are not reduced in size, but tend to differentiate into more mature stromal or mesenchymal tumor types. These findings are particularly useful in bilateral disease to guide treatment decisions, including planning of NSS [18,21,25,26]. After chemotherapy, MRI can also be used to distinguish the active NR and WT (bright on T2-weighted images and STIR sequences) from inactive NR and treated WT (dark on T2 and STIR sequences). Lesions bright on T2 or STIR sequences enhance on T1-weighted images after gadolinium administration while those dark on T2 and STIR do not. The lesions are also suspected to be NRs if preserve oblong or lenticular shape, but this finding is not very sensitive and specific because NRs can also be spherical like WT. Anyway, WT should be suspected when the lesion increases in size, shows heterogeneous enhancement and is spherical [21]. Although DWI cannot differentiate between WT and NBL/ NRs, based on mean ADC values, it can be added to the classical MRI sequences at pre-operative assessment to increase conspicuity for small lesions, both WT and NRs. This is especially important for children who must undergo NSS for bilateral WT, because accurate detection and description of NBL foci may help to optimize surgical resection in order to preserve as much normal renal parenchyma as possible [3,21,24-26]. Finally, in UMBRELLA protocol, tumor volume, measured by imaging after preoperative chemotherapy, has been added as a risk stratification factor for a subgroup of WTs. The volume of the tumor is determined from the largest diameter in the three planes (AxBxCx0.523) using US, but more often the volume is calculated by MRI or CT. If the tumor is large and cannot be delineated from the kidney, tumor and kidney should be measured as a single unit. Patients with a tumor volume of ≥ 500 mL after preoperative chemotherapy and non-stromal and non-epithelial intermediate risk histology and local stages 2 or 3 are treated aggressively (Table 4). This decision is based on analyses of patients from SIOP 2001, which demonstrated that tumor volume is a significant risk factor for poor outcome in this patient group. For patients with stage 1 intermediate risk or any stage with epithelial and stromal subtype, large tumor volume (500 mL) after pre-operative chemotherapy does not significantly affect outcome. Therefore, only patients with Stages 2 and 3 mixed type, regressive type and focal anaplasia will be treated more intensively if their tumor volume after neoadjuvant therapy is larger than 500 mL [1,2] (Figure 10). Imaging for follow-up after treatment.
| Stage 1 | Tumour is limited to the kidney. |
| Tumour is present in the perirenal fat but is surrounded by a fibrous (pseudo)capsule. The (pseudo)capsule might be infiltrated by viable tumour, which does not reach the outer surface. | |
| Tumour might show protruding (botryoid) growth into the renal pelvis or the ureter but does not infiltrate their walls. | |
| The vessels or the soft tissues of the renal sinus are not involved by tumour. Intrarenal vessel involvement might be present. | |
| Stage 2 | Viable tumour is present in the perirenal fat and is not covered by a (pseudo)capsule, but is completely resected (resection margins are clear). |
| Viable tumour infiltrates the soft tissues of the renal sinus. | |
| Viable tumour infiltrates blood and/or lymphatic vessels of the renal sinus or of the perirenal tissue, but it is completely resected. | |
| Viable tumour infiltrates the wall of the renal pelvis or of the ureter. | |
| Viable tumour infiltrates the vena cava or adjacent organs (except the adrenal gland) but is completely resected. | |
| Stage 3 | Viable tumour is present at a resection margin. Nonviable tumour or chemotherapy- induced changes present at a resection margin are not regarded as stage III. |
| Abdominal lymph node involvement is present by either viable or nonviable tumour. | |
| Preoperative or intraoperative tumour rupture, if confirmed by microscopic examination (viable tumour at the surface of the specimen at the area of the rupture). | |
| Viable or nonviable tumour thrombus is present at resection margins of ureter, renal vein, or vena cava inferior (always discuss resection margins with the surgeon). |
|
| Viable or nonviable tumour thrombus, which is attached to the inferior vena cava wall, is removed piecemeal by a surgeon. | |
| Wedge or open tumour biopsy before preoperative chemotherapy or surgery. | |
| Tumour implants (viable or nonviable) are found anywhere in the abdomen. | |
| Tumour (viable or nonviable) has penetrated through the peritoneal surface. | |
| Stage 4 | Haematogenous metastases (for example, lung, liver, bone and brain) or lymph node metastases outside the abdominopelvic region. |
| Stage 5 | Bilateral renal tumours at diagnosis. Each side should be substaged according to the above criteria. |
Table 1: Staging criteria in the SIOP UMBRELLA protocol.
| Pre-treated tumours |
| Low risk |
| Mesoblastic nephroma |
| Cystic partially differentiated nephroblastoma |
| Completely necrotic nephroblastoma |
| Intermediate risk |
| Nephroblastoma–epithelial type |
| Nephroblastoma–stromal type |
| Nephroblastoma–mixed type |
| Nephroblastoma–regressive type |
| Nephroblastoma–focal anaplasia |
| High risk |
| Nephroblastoma–blastemal type |
| Nephroblastoma–diffuse anaplasia |
| Clear cell sarcoma of the kidney |
| Rhabdoid tumour of the kidney |
Table 2: Current SIOP classification of pediatric renal tumors.
| Tumour type | Chemotherapy-induced change | Histological features (% of viable tumour) | ||
|---|---|---|---|---|
| Blastema | Epithelium | Stroma | ||
| Completely necrotic | 100 | 0 | 0 | 0 |
| Regressive | >66 | 0-100 | 0-100 | 0-100 |
| Mixed | <66 | 0-65 | 0-65 | 0-65 |
| Mixed | <66 | 11–65 | 0–89 | 0–89 |
| Epithelial | <66 | 0–10 | 66–100 | 0–33 |
| Stromal | <66 | 0–10 | 0–33 | 66–100 |
| Blastemal | <66 | 66–100 | 0–33 | 0–33 |
Table 3: Histological criteria for Wilms tumour subtyping in SIOP pretreated patients.
| Disease | Tumour volume after preoperative chemotherapy | Treatment | ||
|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | ||
| Low-risk | All | None | ||
| Intermediate-risk, all subtypes | <500 mL | AV (4 weeks) | AV (27 weeks) | AV (27 weeks) |
| Intermediate-risk, stromal or | ≥ 500 mL | AV (4 weeks) | AV (27 weeks) | AV (27 weeks)+flank radiotherapy |
| epithelial-type | ||||
| Intermediate-risk, nonstromal, nonepithelial | ≥ 500 mL | AV (4 weeks) | AV (27 weeks) | AV (27 weeks)+flank radiotherapy |
| High-risk blastemal type Wilms tumour | All | AVD (27 weeks) | HR-1 (34 weeks) | HR-1 (34 weeks)+flank radiotherapy |
| High-risk diffuse anaplasia | All | AVD (27 weeks) | HR-1 (34 weeks) + flank radiotherapy | HR-1 (34 weeks)+flank radiotherapy |
Table 4: Overview of postoperative treatment for localized Wilms tumour in UMBRELLA SIOP-RTSG 2016.
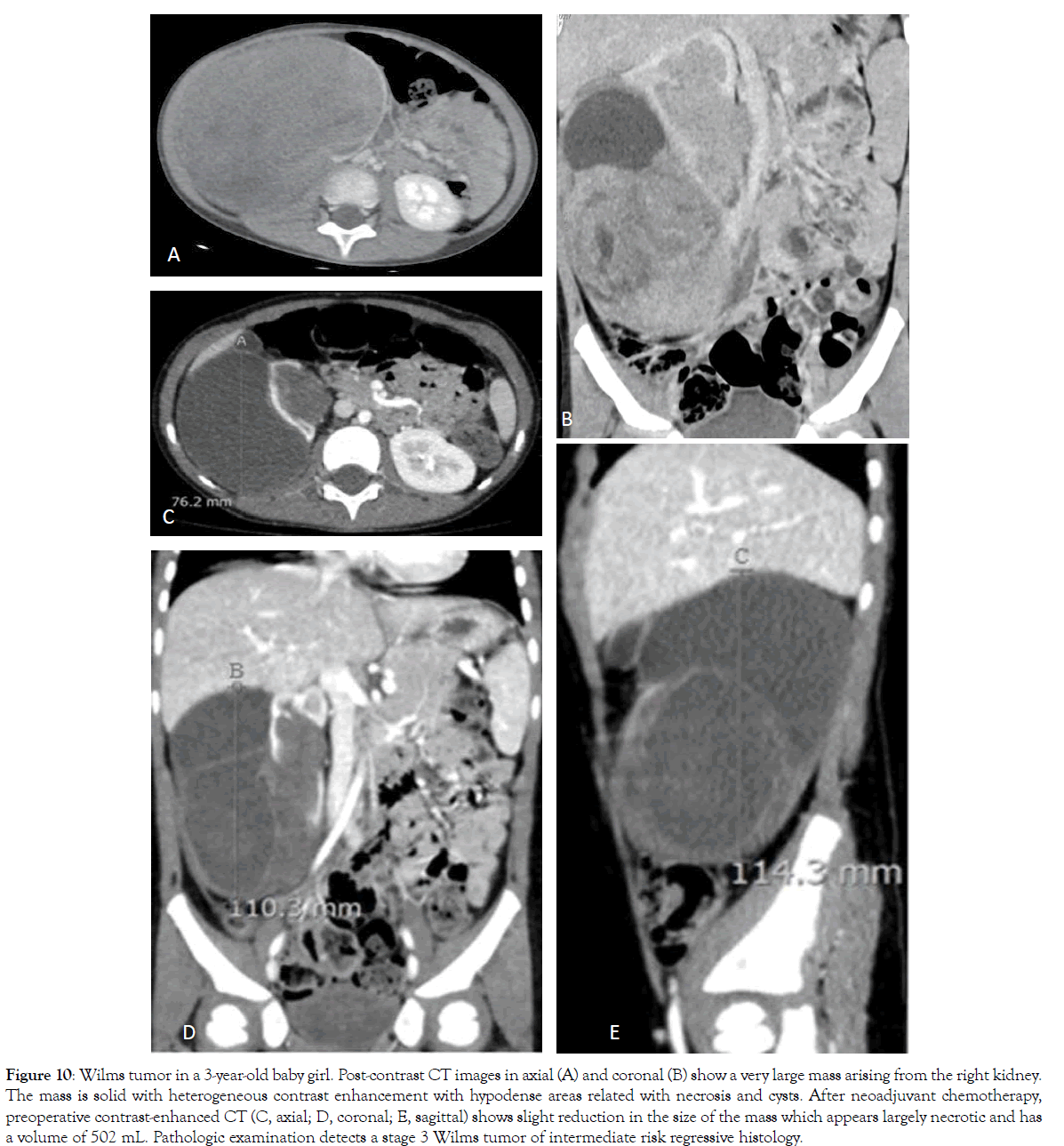
Figure 10: Wilms tumor in a 3-year-old baby girl. Post-contrast CT images in axial (A) and coronal (B) show a very large mass arising from the right kidney. The mass is solid with heterogeneous contrast enhancement with hypodense areas related with necrosis and cysts. After neoadjuvant chemotherapy, preoperative contrast-enhanced CT (C, axial; D, coronal; E, sagittal) shows slight reduction in the size of the mass which appears largely necrotic and has a volume of 502 mL. Pathologic examination detects a stage 3 Wilms tumor of intermediate risk regressive histology.
Recurrences in patients with WT are infrequent and typically occur within 2 years from the time of diagnosis [11,13,20,42]. About 15% of patients with favorablehistology WT and 50% of patients with anaplastic or postchemotherapy blastemal type WT experience recurrence [11,42]. The general profile of relapse site shows that the lungs and pleura alone account for 50%-60%; abdominal recurrences make up to 30% of relapses (isolated abdomen or combined to other sites), while other sites (brain or bone) are involved alone in 10%-15% of cases [13,42,43]. Since children with WT have overall survival rates greater than 90%, follow-up imaging after treatment should be minimally invasive. Moreover, because estimates of overall survival for patients with recurrent favorable-histology WT reach 60%, depiction of recurrences is very important [3,14,20,44]. According to the UMBRELLA protocol, after finishing treatment, all patients should be followed up with chest x-ray (AP or PA and lateral view) and abdominal US every 3 months for 2 years after diagnosis. Patients with high-risk histology (stages 3-5) and intermediaterisk histology (stage 4) have a significantly higher risk of relapse the first year after nephrectomy and should have US/X-ray every 2 months during the first year. Chest CT is performed after the end of treatment, if still persistent disease after neoadjuvant chemotherapy, otherwise X-ray [20,45]. Finally, children with genetic syndromes and a significantly increased predisposition to develop WT are been screened with US throughout the risk period. Scott et al. recommend using US every 3–4 months until 5 or 7 years of age to screen children with a risk of developing WT >5%, depending on the syndrome [5,11,20,45].
For WT, most common renal neoplasm in children with an excellent prognosis, the imaging guidelines are currently defined in UMBRELLA protocol. Radiologists must select non-ionizing and non-invasive techniques such as MRI and must fully exploit the diagnostic potential of the DWI so that children can have the most appropriate treatment plan. When this trial will be closed, the results will show if further steps have been taken in the management and care of children with WT.
The authors declare that they have no conflict of interest.
Author is grateful to families who participated in the study.
This article does not contain any studies with human or animal subjects performed by any of the authors. Informed consent Additional informed consent was obtained from all the patients for which identifying information is not included in this article.
Citation: Brillantino C, Rossi E, Minelli R, Bignardi E, Coppola M, Zeccolini R, et al. (2019) Current role of imaging in the management of children with Wilms Tumor according to the new umbrella protocol. Trans Med 9:206.
Received: 26-Apr-2019 Accepted: 10-May-2019 Published: 20-May-2019
Copyright: © 2019 Brillantino C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.