Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2025)Volume 15, Issue 1
Lipid molecules form spherical micilles in water environment and can entrap biomolecules easily. Phospholipids and cholesterol are main components of our cell membrane. Phospholipids have two long (C12-22) chain fatty acids with hydrophobic nature making ester bonds with glycerol and the 3rd –OH of glycerol connected to a highly charged phosphate group followed by choline or ethanolamine or sugars. Synthetic phospholipid vacuoles or liposomes entrapped transgenes and can transfer biomolecules into nucleus for transcription and further expression in the ribosome. Such gene transfer technologies in human cells were easy and non-toxic delivery of therapeutic genes into nucleus of target organ corrected the defect gene with improvement of health conditions. Such lipid extracellular vehicles or exosomes (20 nm-200 nm) were also produced naturally from membrane of stress-induced prokaryotic as well as eukaryotic cells and were found to involve in various cellular functions transporting miRNAs, DNAs, proteins, lipids and toxins into multiple distal target organs. Recently, exosomes derived miRNAs were investigated in different cancers. Natural micro-RNAs (miR-21, miR-26a) were increased in cancers and antisense to specific miRNA was found to arrest the growth of specific cancer types. Mesenchymal stem cells derived EVs were also found to inhibit certain cancer progression. The pre-micro RNAs were distributed in chromosomes and usually two 20-22 bases processed miRNAs produced from different parts of the 3-D pre-miRNA requiring processing enzymes like Drosha, Dicer and RISC complex. During viral infections, virus particles were also packaged into exosomes to withstand the host immune clearance. Exosomes were purified by ultracentrifugation or gel exclusion chromatography and then drugs, siRNAs and other biomolecules were incorporated into exosomes by electroporation. In this review, the advancement of exosomes technologies and their uses in therapeutic purposes against cancers, heart diseases, diabetes, AIDS, COVID-19, autoimmune diseases and pathogenic microorganisms have been discussed
Extracellular Vesicles (EVs) are ubiquitous membrane-released particles, also called Exosomes. These particles are the smallest (20 nm-120 nm) and are generated from endosomes. Larger vesicles (100 nm-1000 nm) known as micro-vesicles that bud directly from the plasma membrane and finally, apoptotic bodies (ectosomes) generated during programmed cell death (>1 μm) are also known that generate larger EVs. The major components of EVs were phospholipids as shown in Figure 1A and its genesis from cell membrane requiring Dicer and RISC complex was shown in Figure 1B [1].
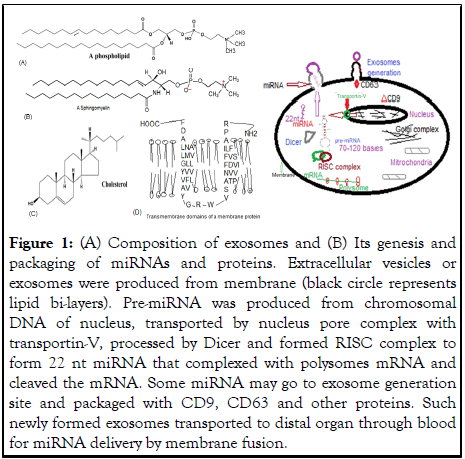
Figure 1: (A) Composition of exosomes and (B) Its genesis and packaging of miRNAs and proteins. Extracellular vesicles or exosomes were produced from membrane (black circle represents lipid bi-layers). Pre-miRNA was produced from chromosomal DNA of nucleus, transported by nucleus pore complex with transportin-V, processed by Dicer and formed RISC complex to form 22 nt miRNA that complexed with polysomes mRNA and cleaved the mRNA. Some miRNA may go to exosome generation site and packaged with CD9, CD63 and other proteins. Such newly formed exosomes transported to distal organ through blood for miRNA delivery by membrane fusion.
Whilst there has been a significant increase in the number publications in recent years, the precise role of EVs in vivo is just beginning to press. The main aim of this review is to provide an update on the natural functions of exosomes and their easy isolation procedure that significantly advanced human clinical trials of therapeutic delivery of antisense, miRNA and drugs to cure many disorders particularly cancers. There are excellent reviews on the role of extracellular vesicles in cellular physiology with proteomic profiling. Our understanding of the biogenesis of EVs, along with selective packaging of biomolecules largely comes from in vitro studies and has been reviewed. Extracellular lipid vesicles are classified by size and density but parameter exclusively varies between experiments and isolation procedures [2].
Isolation of RNA and sequencing
RNA was isolated using miRVana RNA isolation kit (Thermo Fisher). MicroRNAs preparation was performed using the TruSeq small RNA protocol (Illumina) as described by manufacturer, using 1 μg-2 μg RNA and 15 PCR cycles. The miRNA fragments were sequenced on the Illumina HiSeq 2500 system using 50 base pair single read.
Database search
NCBI PUBMED searches (www.ncbi.nlm.nih.gov/pubmed) were done using the words: Exosomes, miRNAs, microRNAs, extracellular vesicles, BEVs, EVs, exosomal miRNAs, stress induced exosomes, exosomes and cancer, miRNAs in cancers. The miRNAs DNA sequences were obtained at www.ncbi.nlm.nih.gov/nucleotide by writing miRNA and human. BLAST searches of miRNA sequences were performed at www.ncbi.nlm.nih.gov/blast. We found homology with individual miRNA at the human chromosomes but homology with mRNAs might not be restricted to Homo sapiens [3].
Isolation of exosomes from animal cell culture
EVs were purified from animal cell culture supernatant. Clarified supernatant (510 × g for 10 min.) was centrifuged at 10,000 × g for 40 min and then passed through 0.22 μ filter and then the supernatant was ultra-centrifuged using sucrose gradient at 100,000 × g for 2 hr at 4°C. Pellet suspended in buffer and checked for integrity by TEM. Different sub-population of exosomes could be separated by Sephacryl S-100 exclusion column chromatography. In another approach, sucrose density gradient (0.4-2.5 M) in HEPES buffer pH 7.4 was used to run at 200000 × g for 16 hrs in SW41 swing bucket (Beckman) at 4°C. The pooled fractions were diluted with 1× PBS and mixed in a rocker for 24 hrs at 4°C. Exosomes were further precipitated by centrifugation at 110000 × g for 1 hr at 4°C.
The dendritic cells were excellent for EVs isolation. Muscle releases alpha-sarcoglycan positive extracellular vesicles carrying miRNAs. EVs were isolated from blood platelets and also purified from urine loaded with biomarkers for cancers. The role of EVs and their purification was reviewed recently. Biomolecules transfer into such lipid carriers usually performed by electroporation. Prostasomes were isolated from prostate cancer cells and contained many miRNAs. Similarly, oncosomes were also isolated from cancer cells with distinct miRNAs like miR-26a, miR-31 and miR-451a. Apoptotic cells derived EVs were larger and had immense roles in cellular physiology. MicroRNAs loaded exosomes were also characterized from HIV infected serum and was quantitatively analysed and purified. Adipose-tissue cells derived exosomes could activate cartilage regeneration [4].
Genesis of exosomes and its components
Exosomes were derived from the membrane endosomal system requiring ESCRT 0, I, II and III along with accessory proteins VPS4 (vacuolar protein sorting-associated protein, isoform 4A), VTA-1 (vacuolar protein-sorting associated protein isoform 1; protein id: NP_057569) and ALIX (ALG-2 interacting protein X; protein id: Q8WUM4). Exosomes act as secretin molecules and natural carriers of lipopolysaccharides, peptidoglycans, proteins, nucleic acids including toxins and virulence factors as well as diverse sRNAs and miRNAs. Many mechanisms for exosomes synthesis were proposed and mainly (i) Budding of endosomes, (ii) Cell membranes blebbing ectosomes and (iii) Decay of dying cells during apoptotic pathways, have been well accepted. The Rab27 and Rab35 GTPases were suggested in the regulation of EVs from endosomes. Thus, EVs act as a secretion mechanism that allows long-distance delivery of active compounds in a protected environment, avoiding direct intercellular contact.
Exosomes contain cholesterol 30%-50%, phosphotydyl choline/ serine/ethanolamine 10%-25% and sphingomyelein (15%-30%).
Other than phospholipids, cholesterol and sphingolipids, tetraspmins components were isolated from exosomes. The CD81 (protein Id: EAX02508), CD63 (protein Id: NP_001244320), CD9 (protein Id: AAC60586) antigens and tumor susceptibility gene 101 protein (TSG101; protein Id: NP_006283) are important cancer biomarkers isolated from exosomes. The Actinin α4, Cyclin Y and Ephrin type-A receptor 2 as well as different oxidoreductases and dehydrogenases were also accumulated in exosomes. The RNA size in exosomes usually varies from 30-150 nucleotides. Although more than 100 genes found in different exosomes, 11 genes were greatly upregulated. These genes are: Glutathione peroxidase 1, zinc finger protein 101, centromere protein Q, small GTPases and ribosomal proteins, B-RAF (Rapidly accelerated fibrosarcoma kinase B oncoprotein; protein id: NP_004324) and EGFR (Epidermal Growth Factor Receptor; protein id: NP_001333826). Such genes might be mutated and DNA fragments of B-RAF gene with V600E mutation were detected in lung cancer cell lines. Many miRNAs were reported in EVs and such more than 1000 miRNAs called a class of naturallyoccurring small non-coding RNAs that usually functioned as specific repressors of protein encoding genes involved in cell proliferation, differentiation and apoptotic pathways. Usually they were produced as preRNA by RNA Polymerase II and then processed into 20-25 nt miRNA via complex pathways involving RNA exonuclease II Drosha, Dicer and ultimately silenced the specific mRNA through RISC complex. The structure of an engineered exosome was shown in Figure 2. Whereas, a classical component of gene-transfer components was described in Figure 3. Such components were very popular earlier days but recently retro-vector mediated gene transfer used human packaging cell line. However, today scientists followed protocol of nano-vesicles for gene transfer experiments. The hairpin structures of miR-26a, miR-631, miR-21 and miR-451a with high melting temperature were shown in Figure 4. The genesis of different miR-26 micro-RNAs and its localization into different human and mouse chromosomes was shown in Figure 5. In Table 1, we presented our BLAST search to locate important miRNAs in different human chromosomes and the adjoining genes that might be affected through mRNA degradation. We also presented the sequence of the miRNAs in Table 1 but data of the BLAST search was not given due to page limit [5].
| Accession no/microRNA | Sequence of the exosome microRNAs that associated in cancer | BLAST homology of the miRNA and human chromosomal localization |
|---|---|---|
| MB031864 miR-631 |
1 gtggggagcc tggttagacc tggcccagac ctcagctaca caagctgatg gactgagtca ggggccacac tctcc 75 | Nei Endonuclease VIII mRNA (AY257544); Ch-15. |
| MA259224 NR_030397 miR-660 |
1 ctgctccttc tcccataccc attgcatatc ggagttgtga attctcaaaa cacctcctgt gtgcatggat tacaggaggg tgagccttgt catcgtg 97 | Chloride voltage-gated channel 5 in Ch-Xq11.23 (NG_007159.3, nt. 95626-95722) |
| NR_029623 MA259188 miR-210 |
1 acccggcagt gcctccaggc gcagggcagc ccctgcccac cgcacactgc gctgccccag acccactgtg cgtgtgacag cggctgatct gtgcctgggc agcgcgaccc 110 | Ras-associated domain family protein 7 mRNA (XM_012930964); Atrophin-1-like mRNA (XM_006877007); Ch-11 (AC138374.2). |
| NR_029629 MN753697 miR-216a-1 |
1 gatggctgtg agttggctta atctcagctg gcaactgtga gatgttcata caatccctca cagtggtctc tgggattatg ctaaacagag caatttccta gccctcacga 110 | Uncharacterized mRNA; Ch-2 |
| NR_029499 Mir-26a |
1 gtggcctcgt tcaagtaatc caggataggc tgtgcaggtc ccaatgggcc tattcttggt tacttgcacg gggacgc 77 | Anti-cancer miRNA Ch-3p21.3 AC105752.2, nt. 104931-105007 |
| PA270683 MN753697 miRNA-423-5p |
1 agctcggtct gaggcccctc agt 23 | Nuclear speckle splicing regulatory protein 1 mRNA (XM_032279323; XM_029484041); Ch-17 (AP023477). |
| NR_030394 miR-657 |
1 ggcaggttct caccctctct aâÂÂÂÂgg 23 | Ch-17(AP023477, nt. 78624816-94);Apoptosis associated tyrosine kinase(NG_029981) |
| NR_029505 miR-31 |
1 ggagaggagg caagatgctg gcatagctgt tgaactggga acctgctatg ccaacatatt gccatctttc c 71 | Interferon epsilon mRNA (XR_002782619) Translation initiation factor IF-2 like mRNA (XM_027088948); Ch-9 |
| NR_029970 miR-451a |
1 cttgggaatg gcaaggaaac cgttaccatt actgagttta gtaatggtaa tggttctctt gctataccca ga 72 | Collagen-ñ2-IVlike (XM_041482734). Era1-like 12S mit. rRNA chaperone 1 mRNA(XM_029519377); Ch-17 |
| NR_029493 miR-21 |
1 tgtcgggtag cttatcagac tgatgttgac tgttgaatct catggcaaca ccagtcgatg ggctgtctga ca 72 | Vacuole membrane protein 1 mRNA (NM_001329398); Ch-17, nt. 57185408-57185337. |
| NR_029635 miR-221 |
1 tgaacatcca ggtctggggc atgaacctgg catacaatgt agatttctgt gttcgttagg caacagctac attgtctgct gggtttcagg ctacctggaa acatgttctc 120 | RNA-binding protein EWS-like mRNA (XM_033412214) Translation initiation factor IF-2 like (XR_006932562). Ch-X. |
| NR_030631 miR-934 |
1 agaaataagg cttctgtcta ctactggaga cactggtagt ataaaaccca gagtctccag taatggacgg gagccttatt tct 83 | Vestigial family protein 1 NG_013270.2, nt. 23727-23809. Ch-Xq26.1 |
| LG164176 NR_029639 miRNA-200b |
1 gtcatcatta ccaggcagta ttag 24 | Tubulin tyrosine ligase like 10 mRNA (XM_040689081). Ch-1; AL390719.47, nt. 106273-106250 |
| NR_031622 miRNA-1290 |
1 gagcgtcacg ttgacactca aaaagtttca gattttggaa catttcggat tttggatttt tggatcaggg atgctcaa 78 | Aldehyde dehydrogenase 4A1. NG_012283; Ch-1p36. |
| NR_029846 miRNA-30e |
1 gggcagtctt tgctactgta aacatccttg actggaagct gtaaggtgtt cagaggagct ttcagtcgga tgtttacagc ggcaggctgc ca 92 | Asperaginase and isoaspartyl peptidase 1; Ch-1 AL354914.9; nt. 34093-34184 |
| NR_029622 miRNA-205 |
1 aaagatcctc agacaatcca tgtgcttctc ttgtccttca ttccaccgga gtctgtctca tacccaacca gatttcagtg gagtgaagtt caggaggcat ggagctgaca 110 | cDNA uncharacterized (BC063704; 1040bp) Ch-1q32.2 |
| NR_029682 miRNA-141 |
1 cggccggccc tgggtccatc ttccagtaca gtgttggatg gtctaattgt gaagctccta acactgtctg gtaaagatgg ctcccgggtg ggttc95 | U47924, nt. 176106-176200 Ch-12p13 |
| NR_029486 miRNA-16-1 |
1 gtcagcagtg ccttagcagc acgtaaatat tggcgttaag attctaaaat tatctccagt attaactgtg ctgctgaagt aaggttgac 89 | Chronic lymphocyte leukemia suppressor locus. Ch-13q14; AF440619, nt. 99683-99595 (+/-) |
| NR_029706 miRNA-185 |
1 agggggcgag ggattggaga gaaaggcagt tcctgatggt cccctcccca ggggctggct ttcctctggt ccttccctcc ca 82 | TANGO2 homolog; Ch-22q11 NG_046857, nt. 21140-21221 |
| NR_029482 miRNA let-7e |
1 cccgggctga ggtaggaggt tgtatagttg aggaggacac ccaaggagat cactatacgg cctcctagct ttccccagg 79 | Sperm acrosome membrane-associated potein 6 (XM_021931395) Ch-19; CP068259, nt. 54779949-54780027. |
| NR_029478 miRNA let-7a-3 |
1 gggtgaggta gtaggttgta tagtttgggg ctctgccctg ctatgggata actatacaat ctactgtctt tcct 74 | Collagen Alpha-1(III) like and Translation factor IF2-like Ch-22; CP068256.2; nt. 46597437-46597510 |
| NR_031757 miRNA-718 |
1 ggccgcggcg cgcaagatgg cggcgggccc gggcaccgcc ccttccgccc cgccgggcgt cgcacgaggc 70 | NG_007107, nt. 122115-122184. Interleukin 1 receptor-associated kinase 1 mRNA. XM_037988836, nt. 1-65; Ch-Xq28 |
| NR_029696 miRNA-127 |
1 tgtgatcact gtctccagcc tgctgaagct cagagggctc tgattcagaa agatcatcgg atccgtctga gcttggctgg tcggaagtct catcatc | Retrotransposon Gag-like 1 mRNA XM_034938161, nt. 3214-3118. Ch-14; NG_045001.2, nt. 25744-25648. |
Table 1: Exosomes mediated miRNA found in different cancers and prediction of their cellular chromosomal origin.
Figure 2: A structure of engineered exosomes.
Figure 3: (A) A recombinant retrovector with insulin gene to be transfer into human; (B) A packaging cell with expressed retroviral gag, pol and env structural proteins and (C) A recombinant retrovirus packaged in packaging cells that can donates insulin gene into human chromosome after infection and reverse transcription into ds-DNA.
Figure 4: Hairpin structures of mir-26a, mir-631, miR-21 and miR-451a with high melting temperature.
Figure 5: Analysis of miR-26. Mature miR-26a was generated from two microRNAs with different chromosomal localization. Pre-miRNA-26a-1 located in Ch-9 and pre-miRNA-26a-2 located in Ch-12 with similarity to mouse miR-26a-2 gene located in Ch-10. The difference of human miR-26a-1 and miR-26a-2 was shown.
Bacterial extracellular vesicles
Bacterial Extracellular Vesicles (BEVs) are spherical lipid bi-layer nanostructures, ranging from 50 nm to 200 nm in size. They are produced by gram-negative and gram-positive bacteria. They are natural carriers of bacterial molecules that include Lipopolysaccharides (LPS), peptidoglycans, proteins, nucleic acids including toxins and virulence factors. EVs act for longdistance delivery of biomolecules in a protected environment and are good signalosome [6].
The composition and content of BEVs varies with the producer strain and also differ depending on the growth phase and isolation conditions. Scientists suggested that membrane remodelling and removal of toxic and unnecessary particles might be the main factors for the generation of EVs in cells.
Stress induction of Stenotrophomonas maltophilia by antibiotic ciprofloxacin caused high release of a heterogeneous population of extracellular lipid vehicles. Deacylation of lipid-A molecules of the outer membrane in Salmonella enterica generated lipid vehicles and mutant bacteria on deacylation enzyme released less such vesicles. Such bacterial lipid vesicles were shown to increase release of the cytokines like INF-γ, IL-1β, IL-6, IL-10, IL-12, IL-18, IL-23, TNF-α and TGF-β during in vivo experiments in animal model. The BEVs was tested for excellent vaccine delivery using CpG as adjuvant.
Proteomic analysis of BEVs from diverse gram-negative pathogens revealed the presence of virulence factors (bacteriocins, virBC) and a large number of proteins (ompA/B/C) involved in biofilm formation, pathogenicity and host-cell interaction. Such lipid vehicles were purified and used in therapeutic purposes. S. aureus derived EVs carrying β- lactamase have been shown to confer ampicillin resistance to Salmonella enterica, Staphylococcus epidermis and E. coli, whereas Acinetobacterium baumannii and Haemophilus influenza derived EVs packaged with β-lactamase (blaTEM gene product) protect group A streptococci from amoxicillin [7].
The Hfq RNA binding protein binds sRNAs (50 nt-200 nt) and thus used to purify sRNAs using antibody to Hfq protein. The sRNA of OmrA/B, ArcZ, DicF, GcvB, MicC/F, Rma, RygA, CyaR, SroC, SraH, etc. have three loops structure and may regulate bacterial pathogenic potential. The Hfq protein also binds to ribosome and inhibits the IS10 transposase mRNA processing in presence of sRNA. In another example, Hfq was shown to bind to a translational enhancer in CirA mRNA and blocked translation. It has been suggested that miRNAs (18 nt-24 nt) function through miRNA-RNA-Induced Silencing Complex (RISC) complex in higher organism but does not exist in bacteria.
Viruses in host cells cause changes in miRNA level likely mediated through EVs. Bellutti, et al. elegantly reported the presence of few Ad5 virus specific miRNAs (mivaRNAI-137) in infected mammalian cells transcribed by RNA Pol-III [8]. The HCMV virus induced changes in mammalian cells was also demonstrated with increased expression of Cytomegalovirus specific miR-US25-1, miR-US25-2-5p and miR-UL112 micro RNAs.
Extracellular vehicles from fungal pathogens
Fungal exosomes are very important for host-fungi interactions. Fungal EVs are carriers of lipids, proteins, toxins, allergens and pigments and have reviewed extensively. Important plant pathogen fungus Botrytis cinerea transports miRNA into plants cells. On the other hand, many cellular interference RNA for virulence genes have also found inside the fungus body purified from plant tissue.
Extracellular vesicles from parasites
Parasite’s survival inside the host cells depends on interplay of extracellular vesicles loaded with biomolecules. Human parasites Leishmania and Trypanosoma excrete exosomes to elicit host IL-8 or IL-10 and evade macrophages where promastigotes form with long tail converted into small and circular amastgotes form for multiplication and survival. Interestingly, Leishmania (Kala-azar) and Trypanosoma (Sleeping sickness) parasites have 10,000 kDNA minicircles (~1 kb) catenated with thirty maxicircles (~32 kb, mitochondrial DNA) and released sRNAs required for processing of mRNAs involved in antigenic variation. A multifunctional metalloprotease GP63 and different heat shock protein 70 were found in EVs acting as virulence factors. Plasmodium falciparum controls RBC physiology by producing EVs that enhance the release of cytokines (IL1, IL6, IL12) from monocytes. Schistosoma japonicum exosomes mediates M1 type immune activity of macrophages. Thus, parasites exosomes have immense role in survival physiology inside the host [9].
Extracellular vehicles from plants
Plants create some EVs and send to fungi pathogens inhibiting secretion of virulence genes. Human plasma extracted extracellular vesicles exhibited a main size of 70 nm, had concentration of 2.5 × 109/ml, and scored positive for tetraspanin CD63, CD81 and annexin II, typical characteristic of the exosome vesicles. Torqueteo Virus (TTV) persists in cells for longer time due to virus incorporation into exosomes. Investigation of plasma EEVs from 122 subjects (37 HIVpositive, 20 HCV infected, 20 HBV infected, 20 kidney transplant recipients and 25 healthy) reported TTV DNA detection in 42 (34%) of the viremic exosome samples (37 were from diseased patients and 5 from healthy people) at a mean level of 4.8 × 103 copies/ml. Polio-virus and Epstein–Barr virusinduced cell lines released exosome were characterized. There is very much advancement in the small RNA genes in genomes after WGS and related computer techniques.
Mechanisms of extracellular lipid vehicles signalling
Extracellular vesicles are known to have a unique signature of lipids, proteins and nucleic acids, reflecting their cell and tissue of origin. Protein composition has been the most extensively studied and EVs are enriched for proteins in the tetraspanin family (CD63, CD9, CD81). Díaz-Garrido, et al. described the mechanisms of microorganisms secreted biomolecules-loaded lipid vesicles modulating host signalling pathways and cell processes [10]. Exosomes was designated as signalosomes with many pharmacological effectors. Adipose cells derived EVs attenuates cartilage degeneration. Antibacterial activity of prostrate derived EVs was reported. Let-7 miRNA was designated as tumour suppressor. MiR-221 and miR-222 inhibit normal erythropoiesis and erythroleukemic cell growth via Kit receptor down regulation. Frequent deletions and low expression of miRNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia was detected. Metastatic CRC cells was shown to release more miR-181a-5p-rich EVs than normal cells. Packaged miR-181a-5p into CRC EVs activated hepatic stellate cells activating the IL6/STAT3 signalling pathway. Such activated cells were involved in secreting the chemokine CCL20 activating a CCL20/CCR6/ERK1/2/ Elk-1pathways. Clinically, a high level of serum extracellular lipid vehicles containing miR-181a-5p was correlated with increased liver metastasis in CRC patients providing novel insight into the mechanism of liver metastasis.
Preparation of synthetic lipid vehicles and gene transfer technology
A significant enhancement of retroviral gene transduction efficiency was observed depending upon the type of lipid formulation like DOSPA:DOPE>DOTMA:DOPE>DOTAP, resulting in 60, 37 and 5-fold increases in transduction efficiency, respectively. Exosomes contain chloresterol 30%-50%, phosphotydyl choline/serine/ethanolamine 10%-25% and sphingimyelein (15%-30%). Cholesterol content of prostasomes was very high (~53%) and use of such EVs might be avoided.
Liposomes are available commercially for gene transfer experiments using cells, recombinant plasmid retrovectors and human and animal packaging cell lines. Lipofectamine reagent may be available from GIBCO-BRL. Plasmid vectors may be made or commercially available which contained a strong eukaryotic promoter-enhancer, ribosome entry site, retroviral packaging signal and a gene for neomycin (neo) or hygromycin (hyg) or puromycin (pac) resistance. A therapeutic gene (insulin or adenosine deaminase/growth hormone/Interleukin-2) was inserted into vector as required. A large-scale recombinant plasmid was isolated by CsCl density centrifugation and then sequenced by Sanger di-deoxy method for confirmation and authenticity. About 20 μl lipofectamine reagent, 20 μg recombinant plasmid DNA were mixed to make exosome-like micelles and added into packaging cells (60% confluency, T-25 flask or 10 cm plate) grown in DMEM medium in presence of 5% CO2 with humidification at 37°C temperature. Then, the cells were selected with G418 antibiotic and expanded in T-75 flasks for virus purification from culture supernatant. The recombinant retrovirus was purified by PEG precipitation and clarified by steps glycerol-density gradient at 60000 rpm for two hrs in an ultracentrifuge at reduced pressure and temperature. Such recombinant virus was injected into human organ for gene delivery into chromosome. Retrovirus RNA genome first converted into ds-DNA by reverse transcriptase and then mobilized into chromosome by cellular recombinases. MMLV/CMV/VL30/Adenoviral promoters were used but house-keeping gene promoters (Glyceraldehyde 3-phospahate dehydrogenase, thymidine kinase) were also used. The efficiency of promoter could be monitored for one year by assaying the expression of proteins in target organ or simply in blood using ELISA technique. The process is very simple and efficient. Thus, similar procedures were adapted for exosomes and then used for gene transfer and biomolecules delivery. An engineered exosome contained many components (Figure 2). The use of liposomes in gene therapy technology facilitated through retro-virus particles but now-a-day nanotechnology procedures were followed (Figure 3). Thus, recent trend is to use natural exosomes in human gene transfer experiments instead synthetic liposomes [11].
Engineered exosomes for the treatment of different cancers
The rapid surge of cancer morbidity and mortality worldwide has shown more than ~20 million new cancer cases and an estimated 10 million deaths in 2020 and an expected ~30 million cases in 2050. Metastasis cancers were associated with mutated and over expressed oncogenes and supressed tumour suppressor genes. Exosomes derived from cancer cells depend on cell-surface heparin sulphate proteoglycans for their internalization and function. The roles of exosomes in cancer progression were studied by different workers. Studies indicated that exosomes secreted by B lymphocytes contained histocompatibility class II antigen proteins, which could induce the immune response of T lymphocytes. Such lipid carriers also carry miRNAs with potential application for cancer therapy. There are over 20000 publications in the database relating cancer gene therapeutics using extracellular lipid vehicles. Engineered exosomes with miRNA, siRNA, drugs have been developed and used in clinical therapy. Such lipid vehicles carried makers (e.g. miRNAs) for cancer in sense or antisense to control oncogene and interleukin expression. An efficient exosome (1011 particles/ml) pakaging of miR-26a, miR-31 and miR-451a anti-tumor microRNAs by electroporation (250 V with 125 μF) was shown by different workers and such engineered exosomes induced apoptosis in HepG2 liver cancer cell lines. Salivary microRNAs miR-let-7a-5p and miR-3928 was found as potential biomarkers for head and neck squamous cell carcinoma and a good hope for antisense therapy. Exosomal microRNAs like let-7d-3p and miR-30d5p were also found as diagnostic biomarkers and therapeutic potential for cervical cancer. On the contrary, miRNAs were shown to induce cancer metastasis where Antisense-miRNA Oligonucleotides (AMOs) might be an important approach to treat cancer. Han and coworkers have shown that AMOs of miRNA-221 were reduced the colorectal cancer. There was a report that miR-33a-5p suppressed colorectal cancer cells growth by inhibiting MTHFD2. Such method also used for doxorubicin (1 mM-2 mM) delivery into exosomes for cancer therapy. The miR-34a, miR-34b and miR-34c, have emerged as the most extensively studied tumor-suppressive miRNAs and novel therapeutics against many cancer types were under clinical trials.
Dendritic cells exosomes engineered with Lamp2b iRGD peptide plus doxacycline gave good therapeutic index with no toxicity for the treatment of breast tumor. Similarly, IL4 plus GM-CSF delivery was also shown quite satisfactory with activation of many immune effectors during phase-1 study of lung cancer. Similarly, kidney derived exosomes engineered with Lamp2b IL3 plus Imatinib or BCR-ABL siRNA controlled the chronic myeloid leukemia in vivo. Further, MiR-621 was found to be up-regulated in lymphoma and leukemia (Lung cancer derived exosomes engineered with Rab27a prevent adenocarcinoma that was grown in a pre-established model. Targeting K-ras with exosomes miRNA drastically reduced the pancreatic tumor). The MiR-142-3p was found as a potential prognostic biomarker for esophageal squamous cell carcinoma. Baek, et al. reviewed the biological roles of immune cell-derived EVs for cancer therapy [12]. The authors compare EVs derived from immune cells including dendritic cells, T cells, naturalkiller cells and macrophages for EV-based cancer therapy applying cytokine-stimulated cells or antibody-decorated EVs. Similar approach was done with antisense miRNA of STAT3 using micro-vesicles to alleviate liver fibrosis. The miR-934 microRNA did enhance tumour metastasis establishing a role of such RNA in cancer but also anti-miRNA (antisense miR-934) therapy could be developed. Similarly, tumour of pancreas could be targeted by antisense to miR-155.
Wang, et al. performed an elegant method of tumor therapy in mice utilizing both nanotechnology and gene transfer [13]. In this method, biotinalised isolated exosomes loaded with doxorubicin drug was first made and coated with polydopamine magnetic Fe2O4 nanoparticles which was fixed with avidinmiR- 21 to target cancer cells. Such particles during their targeted journey into tumor surface induced heat energy by IR light, killing cancer cells and at the same time such heat energy broke the exosomes-barrier releasing doxorubicin further killing cancer cells. In animal model tumor size was reduced to over 97% demonstrating the utility of such approach in future human cancer therapy.
Exosomal miR-21 and miR-29a target mRNA for degradation but also bind to Toll-Like Receptors (TLRs) and activate immune cells. Similarly, breast cancer cell derived miR-105 reduced ZO-1 gene expression in endothelial cells promoting metastases to the lung and brain. The exosomal miRNAs like miR-2, mirR-let-7a, miR-1246, miR-150, miR-21, miR-223 and miR-23a, may be regarded as the diagnostic biomarker of colorectal cancer. Similarly, miR-21a, mir-27a, mir-17-5p, miR-125a/b, miR-335 may be regarded as the breast cancer miRNAs. The miR-122 could suppress glucose uptake in nontumour cells in the premetastatic niche facilitating the tumour progression. The exosomes derived from breast cancer cells contain Dicer, AGO2 and TRBP and activated the processing pre-miRNA into mature miRNA for gene silencing in target cells leading to tumour formation. The EVs of pancreatic and breast cancer cells contained high concentration of Glypican-1 (GPC1) which was also isolated from exosomes of those cancer cells cultured supernatant and used as diagnostics with high specificity. Further, few tumour suppressor genes were reactivated by miR-26A1 via enhancer reprogramming in NSCLC whereas important tumour suppressor gene p53 activated miR-34. Similarly, reports suggested that NF-kβ reactivated by mir-21, miR-155, miR-146, Let-7a/b miRNAs whereas other important TFs like STAT3 and MyoD controlled the genesis of miR-21and miR-133 respectively. Exosomes found good promise for the inhibition of brain tumour and oral cancers.
Clinical trials (NCT01854866, NCT02657460, NCT03608631) are undergoing using encapsulated methotrexate, cisplatin or KrasG12D siRNA to different cancer patients. Plant-derived exosomes also has many applications as nano-drug carriers. In a clinical trial for COVID-19 disease, exosomes have also tested in clinical trial to reduce the cytokine storm (NCT05216562) [14].
Roles of exosomes in cardiovascular diseases
Death due to Cardiovascular Diseases (CVD) like Acute Myocardial Infraction (AMI), ischaemic heart disease, arrhythmias, heart failure and left ventricular hypertrophy are increasing worldwide. The miR-1254, miR-1306, miR-208b, miR-499, miR-210 etc. were increased in heart failure patients whereas miR-23a, miR-26a, miR-150 were increased in arrhythmia. The miRNAs like miR-1, miR-194, miR-328, miR-499, miR-21 and miR-223 etc. were increased in AMI patients with ischemia-related heart failure. Circulating miR-499 was suggested an early biomarker for myocardial infraction during cardiac surgery and the level of miR-499a decreased significantly after on-pump CABG surgery. The miR-208a is also an early AMI diagnostic marker as also miR-133a/b whereas miR-150-5p was down-regulated. The miR-146a-3p microRNA containing exosomes released from human Cardiac Progenitor Cells (CPCs) are cardio-protective and improved cardiac function after myocardial infarction through remote ischaemic preconditioning. Progression of atherosclerosis could be happened with increased expression of miR-133, miR-208a, miR-146a/b and miR-106b. Cardiac inflammation could be regulated by exosomes derived from Krüppel-like factor 2 induced endothelial cells during dilated cardiomyopathy. Diabetic cardiomyocyte-derived exosomes loaded with miRNAs like miR-320-3p and miR-3a regulated angiogenesis on endothelial cell cultures and might be important for the treatment of diabetes-caused ischemic cardiovascular disease.
Roles of exosomes in diabetes
Nine miRNAs (hsa-miR-27a, hsa-miR-29b, hsa-miR-126, hsamiR- 142-3p, hsa-miR-142-5p, hsa-miR-144, hsa-miR-199a-5p, hsamiR- 342-3p and hsa-miR-1307 were found elevated in type-1 and type-2 diabetes mellitus. While microRNAs like miR-3383p and miR-342-3p only expressed in type-1 diabetes and miR-30b and miR-144, miR-140-3p highly expressed in type-2 diabetes. However, miR-342-3p was very much repressed in both type-1 and type-2 diabetes. It was proved that expression of miRNA-29 in pancreatic β cells enhanced inflammation and diabetes via TRAF3. Also, elevated expression of miR-22-3p was shown to impair gluconeogenesis by silencing Wnt-responsive transcription factor Tcf7. Hepatic miR-22-3p expression impaired gluconeogenesis by silencing the Wnt-responsive transcription factor Tcf7 implicating its role in the treatment of diabetes. The Mir-690, an exosomal-derived miRNA from M2- polarized macrophages, improved insulin sensitivity in obese mice. Exosomal nanoparticles with microRNA-375 were shown to repair metabolic syndrome like diabetes. The exosomes from blood of diabetic patients carry more miR-20b-5p than nondiabetic patients and thus a new therapeutic application could be designed. Overexpressed miR-20b-5p reduces AKITP concentration and the effect of insulin on glycogen accumulation in muscle cells. The targeted delivery of miR21, miR-27a and miR-146a exosomes may be beneficiary to diabetes induced neuropathy targeting and lowering the effects of RhoA, PTEN, SEMA6A and NF-κB factors.
Wu, et al. demonstrated that miR-130a-3p encapsulated by liver cell exosomes could reduce a specific phosphatase 2 (PHLPP2) expression to activate AKT-AS160-GLUT4 signalling pathway in adipocytes increasing GLUT4 expression and further alleviates abnormal glucose metabolism [15]. Wang, et al. studied exosomes from pancreatic cancer cells carrying miR-883b-5p, miR-450b-3p and miR-251-3p reduced GLUT4 expression by regulating PI3K/AKT signalling pathways inducing insulin resistance [16].
Roles of exosomes in AIDS progression and therapy
HIV virus is the main etiology for AIDS progression as pro-viral DNA genome integrated into host genome and indefinitely could be their releasing mRNA for virus assembly. During the different life cycles of HIV virus, exosomes lipid particles play an important role where it may help HIV transmission and in contrary, engineered exosomes may be used for the elimination of latent HIV virus from the CD4+ T-lymphocytes. Milk derived exosomes from HIV infected mother contains distinct miRNAs and used as diagnostics marker for AIDS. Exosomes were also isolated from plasma of AIDS patients and was thought to help the disease progression. Exosomes packaged with an engineered zinc-finger protein fused with a DNA methyltransferase 3A mRNA could block HIV propagation in a humanized mouse model. Here, zinc-finger domain helped to bind HIV promoter with total repression of HIV life cycle. Increased proinflammatory cytokines and chemokines (IL1α, IL2Rα, IL5, IL&, IL9, IL16, IL19, etc. including interferon IFNα2, CXCL10, CCL2/3/4) in EVs of human plasma of HIV-1 infected individual was reported. HIV virus was found in human semen extracellular vehicles (SEVs; ~30 nm-200 nm) at a range 1011 particles/ml of semen as well as different protein markers loaded with SEVs like ALIX and HSP70 cancer markers.
Roles of exosomes in COVID-19 progression and therapy
Exosomes were suggested as mediator of SARS-CoV-2 disease progression in human as it carried receptors like ACE-2 and CD9. Corona virus is a ~30 kb sense RNA virus which expresses ORF1ab polyprotein (7096 aa) that is cleaved into sixteen polypeptides with diverse functions like RNA-dependent RNA polymerase, RNA helicase, RNA topoisomerase, proteases and methyltransferases as well as accessory proteins. The expression of miR-17, miR-29, miR-31 and miR-126 in serum of COVID-19 patients were observed. However, in other studies miR-31-3p, miR-19-3p and miR-210-3p expression were unchanged but miR-193-5p was slightly increased. Exosomes from serum of COVID-19 patients contained Tenascin-C and Fibrinogen-β proteins that were shown to produce inflammation signals in distant organs. Protease inhibitors and immunosuppressive and antiviral drugs (Tocilizumab, Lopinavir, Remdesivir) may be used in micro-vehicles cargo for COVID-19 therapy in the future. A corona virus genomic miRNA like sRNA (CoV2-miR-07a-3p; 52 bases) was found in serum of COVID-19 patients that was originated from orf7a region. The sequence of the miRNA was reported as 5’- UUUUCUUGGCACUGAUAACACUCUACUUGTGAGCU UAUCACUACCAAGAGTG-3’ that formed a loop structure with ΔG=-19.7 Kcal/mole. Incorporated protease inhibitors in exosomes was found successful for COVID-19 therapy [17].
Roles of EVs in neurological disorders
Old neurological disorders like Parkinson’s and Alzheimer’s affect million people worldwide. In Parkinson’s disease, the α- synuclein protein (Protein Id: EAX06035) was accumulated in neuron cells and also aggregated causing damage of neuron. It was found that much α-synuclein protein transport was mediated by extracellular vehicles. Alzheimer’s disease was caused by the deposition of β-amyloid protein (Precursor beta-amyloid; Protein Ids: P05067 and NP_005157) and hyperphosphorylation of tau protein (Protein Id: AAC04279). β-amyloid and tau proteins fragments were also found in the extracellular vesicles isolated from neuron cells of brain. Exosomes mediated transport of biomolecules into brain was successful improving many neurological disorders. Animal model study suggested miR-132, miR-137, miR-222 and miR-221 might be expressed in MECP2- null neural cells. The BDNF gene was repressed by increased levels of miR-30a in brain but miR-381 and miR-495 microRNAs had also some regulatory roles in the brain physiology. The Xlinked Methyl-CpG-Binding Protein 2 (MECP2) binds to methylated CpG island of genes and also control BDNF gene transcription. The miR-718 was located in the downstream of human MECP2 gene but miR-718 appeared in primate interleukin-1 receptor associated protein gene 5’ upstream implying many microRNAs might be involved in neuron development [18].
Exosomes in autoimmune disorders
Exosomes containing miRNAs were detected in autoimmune disorders or chronic inflammatory diseases. The miRNA hsamiR- 142-3p has been implicated in psoriasis whereas hsamiR- 150 and hsa-miR-142-5p microRNAs were linked to lupus nephritis. Exosomal miR-21 and miR-155 were up-regulated (p<0.01), whereas miR-146a expression (p<0.05) was downregulated in patients with systemic lupus erythematosus. Crohn’s Disease (CD) is a chronic inflammatory disease of the gastrointestinal tract where a 3-fold increase in serum exosomal miR-144-3p levels was reported in patients with CD compared with those in healthy controls. Over expression of miR-595 and miR-1246 in the sera of patients with active forms of inflammatory bowel disease were also observed. The miR-150 was differently expressed by immune cells at various stages of their maturation and differentiation and might be involved in the pathogenesis of allergic and autoimmune disorders. The miR-150 functions were mediated by targeting Myc oncogene. The Hsa-mir-144-3p expression was increased in umbilical cord serum of infants with atopic dermatitis. The miR-181a, miR-146a and miR-146b in spleen CD4+ T lymphocytes had pro-inflammatory roles in a murine model of asthma.
Problems of gene therapy using retroviral vector and packaging cells lines were understood and such problems voided the success of gene therapy protocols in human during 1970’s. Main success of EVs technology resides to less toxicity profiles and biocompatibility of such lipid vesicles. During viral infection, host cells trigger response by releasing specific miRNA. Bacteriaderived miRNAs were less explored but 5’-GUC GAG GUC UUU CAG AUG GAT GGC GCG UAG GAA CCA UGA UCG GAA GAG CUC CAC C-3’ sRNA from Mycobacterium smegmatis was found in serum of patients. Importantly, miRNAs from animal and human cancer cells advanced the knowledge of cancer biomarker whereas CAR-T cells derived exosomes have potent antitumor activity with low toxicity profiles to normal cells. The PinT, a bacterial sRNA carried by exosomes acted as a master regulator of virulence in Salmonella typhimurium during infection in human and also a similar mechanism mediated by ProQ protein in Enterobacteria.
Lee and Yoo have introduced the mesenchymal stem cells derived engineered EVs (Figure 2) loaded with active pharmaceutical ingredients targeting NF-κB and TGF-β pathways in kidney diseases [19]. Kang and Kwon described the potential use of nave EVs derived from various cell sources such as MSCs, cardiac progenitor cells, induced pluripotent stem cells and somatic cells from heart. Choi and Shin described the potential applications of engineered EVs for excessive inflammatory responses associated with severe COVID-19 diseases [20].
EVs loaded with miRNAs used in therapeutic application of cancers. Many miRNAs were transcribed from the regions of oncogenes and tumour suppressor genes. Use of designer microRNAS has great promise in health application. Many miRNAs genes were located in the frazile sites of cancer genes but also distributed in different chromosomes. A details method of modification of miRNAs was described for clinical application of exosomal microRNAs. DNA as well as RNA gene transfer using exosomes instead liposomes into human was initiated recently [20].
We see that EVs (Exosomes) play an important role in next generation gene therapeutic technologies. Exosomes deliver active biomolecules to target cells and have vital functions in several pathological processes showing promise to novel treatment strategies for many diseases. As we discussed, targeting different cancers is the main focus today specifically using EVs loaded with sense or antisense miRNAs. On the other hand, cancer biomarker discovery was accelerated from EVs studies giving a new platform for new drug development. We foresee a golden use of exosomes technology in the future. The database of mammalian extracellular vesicles (i.e., exosomes and ectosomes), EVpedia (http://evpedia.info), has discussed the high-throughput data for systematic analyses of extracellular vesicles and the intravesicular protein-protein interaction network analyses of mammalian extracellular vesicles. However, database has too many miRNA sequences and their functional roles in different cancer cell lines seem to be contradictory. Most of the mir genes are intragenic (70%), but some are transcribed from the noncoding area of chromosomes (30%) as independent transcription units. Thus, better classification of different exosomes and their miRNAs may be the future goal to get utility of exosomes research. Further common platform for bacterial, animal and plant sRNAs, siRNA and miRNAs may be addressed carefully to know exosomes functions. Similarly, universal approach for the isolation of exosomes may be commissioned so that their inclusion bodies found to be optimize to discover target therapeutics more easily.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Chakraborty AK (2025) Current Exosomes Research in Cellular Physiology, Disease Diagnostics and Therapeutic Biomolecules Delivery. Trans Med. 15:339.
Received: 29-Dec-2023, Manuscript No. TMCR-23-28840; Editor assigned: 01-Jan-2024, Pre QC No. TMCR-23-28840 (PQ); Reviewed: 15-Jan-2024, QC No. TMCR-23-28840; Revised: 16-Jan-2025, Manuscript No. TMCR-23-28840 (R); Published: 23-Jan-2025 , DOI: 10.35248/2161-1025.25.15.339
Copyright: © 2025 Chakraborty AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.