Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Commentry - (2018) Volume 9, Issue 6
I have read the cited article [1] thoroughly. In Section 2.1 Sampling, as stated in the manuscript, the water samples were immediately acidified with HCl (Hydrochloric acid) after filtration. This is an incorrect approach. Since the determination is to be carried out by laser fluorimetry, the addition of HCl will further increase the chloride ion concentration, which is a well-known quencher of uranyl fluorescence [2]. HCl is never used for acidification of water samples for preservation. Nitric acid (HNO3) is always recommended for acidification. It is further stated in Section 2.1 that, for chemical analysis, the water samples were collected in suitable bottles without the acidification step, and the pH and Total Dissolved Solids (TDS) were monitored with a multi-parameter TDS and pH meter. I would request the authors of the paper for providing a full statement of the measurement protocol used.
Laser fluorimetry is a versatile technique for the direct determination of uranium in natural water samples [2,3]. In highly saline water samples, the standard addition method will not work, and it requires removal of the chloride ion by treatment with potassium persulfate (K2S2O8). In the recommended procedure, potassium persulfate (K2S2O8) is added to the sample, which is then heated to dryness to drive off the chloride ion as chlorine. The residue is taken up in water and neutralized before being made up to volume and analyzed with the UA-3 using standard addition methods [2]. The traditional laser fluorimetry method used by the authors is not clearly stated from sampling to analysis [1]. The concentration levels of chloride ion present in water samples having high TDS should be stated.
As stated in Section 2.2, a limited development in the method was implemented through a pretreatment of the water sample including uranium coprecipitation with calcium phosphate, dissolving and digesting of the precipitate with nitric acid and a few drops of H2O2 and maintaining the final 5 mL sample volume solution at pH near 7 (of course chloride-ion quench correction will not be needed following this procedure).The procedure details, tolerance limits and advantages of the proposed developed method [1] over the recommended procedure [2] have not been mentioned and discussed in the cited manuscript [1]. Any additional chemical preparation of sample will introduce contamination and result in a high blank value. Moreover, co-precipitation procedures are time consuming and are never recommended [2]. Direct methods for the analysis of uranium in natural water samples should be adopted.
In most samples [2], there is some quenching of uranyl fluorescence relative to distilled or deionized water. The method of standard addition to the sample is commonly adopted for correction of uranyl quenching in the water sample. The amount of standard addition should be roughly related to the unknown value in the sample. The volume of standard added is kept small so that the total volume is not affected, and the calculation is simplified. The increase in concentration in the cuvette on the addition of a standard addition is given by:
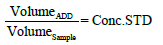
Moreover, the method of standard addition, (say, one addition, two addition, three additions of 5 μL of 1 ppm standard in a 6 mL sample may be used for standardizing the instrument response by using distilled or de-ionized water as a blank sample. The calibration by the standard addition method avoids the need for the preparation of very dilute standards with their stability problems.
As stated in Section 2.2, the details of the measurement protocol are given elsewhere, citing Reference Number 11. What is the purpose of citing this reference? Have the authors read this publication? The authors are advised to read the procedures [2] and Reference Number 11 cited in manuscript [1] carefully.
From the Article’s Figures 2 and 3 and Tables 2 and 3, there is no clear advantage of the proposed modified laser fluorimetry method in comparison with traditional laser fluorimetry and also other methods.
It is suggested that the to the authors should update their understanding of the published literature and discuss the significant advantages of the modified procedure explicitly in comparison with over the traditional laser-induced fluorimetry and other recommended procedures in the literature [2].
A comparative performance aspect of the analytical protocol will be more useful for the researchers especially for the reliable determination of low uranium concentration in high TDS water samples.