Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2017) Volume 8, Issue 4
The experiment was conducted to find out potential of insecticidal plants combination against Acanthoscelidesobtectus through identifying the best potency of combinations and determining dosage rates. Leaf and seed powders of six insecticidal botanical plants, namely Jatropha curcas(L.), Allium sativum(L.), Citrus aurantifolia(L.), Eucalyptus globules (L.),Euphorbia tirucalliand Vernonia amygdalina Del.were mixed to 1% and 2%w/w binary formulations. The synthetic insecticide primiphos-methyl at the rate of 0.1/100 gm grain dust and untreated grains were used as positive and negative controls, respectively. High dosage rate of binary formulations (2%w/w) had better toxicity (high adult insect mortality) than low dosage rates formulations (1%w/w). Combinations of botanical powders showed highest adult mortality, F1 progeny reduction and lowest weevil perforation index and weight loss comparable to untreated control. Allium sativumwith Jatropha curcascombinations had best efficacyamong the botanical combinations at both dosage rates of treatments. Every treatment combined with Allium sativumhad better efficacy than the rest formulations.
<Keywords: A. obtectus; Binary formulation; Botanical insecticides; P. vulgaris
Common bean is one of the major food and cash crops in Ethiopia and it has considerable national economic significance. It is often grown as cash crop by small scale farmers and used as a major food legume in many parts of the country where it is consumed in different types of traditional dishes [1]. The area devoted to common bean production in Ethiopia is 3,59,235 ha-1 with a total production of 0.41 M tons and average yield of 1.2 t/ha, CSA (Central Statistics Agency of Ethiopia 2012). It is mainly grown in eastern, southern, south western, and the Rift valley areas of Ethiopia [2].
The common bean was originated in tropical America (Mexico, Guatemala, and Peru) but there are also evidences for its’ multiple domestication with in Central America [3]. The crop is now widely distributed throughout the world and is grown in all continents except Antarctic and occupies more than 90% of production areas sown to Phaseolusspecies [4].
The world demand for common bean is highly increasing because of its significance to human nutrition as a source of proteins, complex carbohydrates, vitamins, and minerals. Its importance in reducing blood cholesterol level and combating chronic heart diseases, cancers and diabetics is also gaining recognition from human health point of view [4,5]. Pre-and post-harvest damage by insect pests, inter alia, is a major limiting factor of bean production. Especially in smallholder farming conditions, under which most beans are grown in the region. Stored beans suffer heavy losses in terms of both quality and quantity mostly by bean bruchids [6].Acanthoscelidesobtectusis the one of major bean weevilspecies of bruchids attacking stored beans, causing yield losses reaching up to 38% [7,8].
To reduce storage losses due to insect pests, synthetic insecticides have been recommended. However, their use is limited under small scale farming condition due to high costs and infrequent supply [6,9]. Besides, indiscriminate use of insecticides may result in undesirable consequences such as resistance development by the pest, secondary pest outbreaks, wide spread environmental hazards and risk to spray operators [10,11]. For these reasons, development of other alternative control methods such as botanical insecticides have gained significant importance in bruchid management [8,12,13]. Use of botanical insecticides not only confers effective pesticidal effect against bruchids but also serves as ecologically sound and economically feasible control option with low health risks to consumers [9,10,14]. Different plant extracts may act synergistically to effectively inhibit pest growth and developments compared with a single constituent extract and development of pest resistance is less likely when used over time [15-17].
However, there were investigations of alternative botanicals powders to minimize damaging of common bean that was not sufficient due to effectiveness of potency so; there is limited information about combine effect of botanical powder against bruchids.The current study was conducted to evaluate combinations of basic botanical formulations with the objective of enhancing effectiveness of constituent botanical in mixtures and reducing dosage rates. The botanical insecticides parts used in this study are shown in Table 1.
| No. | Scientific name | Common name | Parts used |
|---|---|---|---|
| 1 | Allium sativum | Garlic | Leaf |
| 2 | Citrus aurantifolia | Lime | Leaf |
| 3 | Eucalyptus globules | Tasmanian blue gum | Leaf |
| 4 | Euphorbia tirucalli | Milk bush | Leaf |
| 5 | Jatropha carcus | Physic nut | Seed |
| 6 | Vernonia amygdalina Del. | Bitter leaf | Leaf |
Table 1: Name of Botanical plants and parts used against Acanthoscelides obtectus.
Insect rearing
Bean bruchids (Acanthoscelidesobtectus) were obtained from laboratory culture reared on disinfested common bean variety. The experimental insects were maintained under laboratory condition (27 ± 3°C, 60 ± 10% RH, 12L: 12D) at Melkassa Agricultural Research Center (MARC) (8°24′N; 39°21′E). The food medium of bean seeds used for insect rearing was first disinfected by keeping the grains in the oven at 40°C for 4 hours and allowed to cool for 2hrs before use [18]. Infestation was done by introducing 100 parental adults (1:1 sex ratio) in 1L volume of glass jars containing 250 g of bean grains. The parental adults were sieved off 15 days after oviposition period and the grains were kept under laboratory condition until the emergence of F1 progeny. New generations of adult bean bruchids (Acanthoscelidesobtectus) obtained from this culture were used in the experiment.
Plant materials and treatment formulations
Plant parts (leaves and seeds) of the botanical plants E. globulus,A. sativum, C. aurantifolia,E. tirucali, J.curcasandV.amygdolinawere collected from MARC and the surroundings. The plant materials were air dried and crushed separately into fine powder using a pestle and mortar. The resultant powder was further sieved through a 0.25mm mesh to obtain a fine dust. The powders were weighed into 0.5 and 1 gm-samples and then mixed appropriately to constitute binary formulation at either 1 or 2%w/w admixture on 100gm bean samples.
Toxicity assessment
Well disinfected common bean seeds (100 gm) treated with various binary formulations of botanical insecticide powders were placed in the 1L volume glass jar. The glass jars tops were covered with nylon mesh to allow aeration and held in place with rubber bands. The effectiveness of treatments was assessed by introducing 7 pairs of 3 days old bruchids (obtained from laboratory culture) to the treated and control grains. The synthetic insecticide primiphos-methyl at the rate of 0.1/100 gm grain dust and untreated (control) grains were used as positive and negative controls, respectively. Percentage of mortality was calculated using Abbott’s formula by counting number of dead insects in each jar 24hrs, 48hrs, 72hrs, and 96hrs after treatments and adult insects introduced in glass jars each contain 100g bean seed [19]. Adult insects were considered dead when no response was observed after probing them with forceps. At the end of each assessment, dead insects were removed. The experiment was arranged in completely randomized design (CRD) with three replications.
Abbot’s formula: 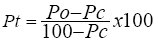
Where Pt=Percent (%) mortality; Po=Observed mortality; Pc=Control mortality.
Effect of powders on F1 progeny
After toxicity assessment of plant powders, remaining A. obtectusadults on treated and untreated jars were kept for additional 10 days and were sieved and discarded (both live and dead). The infested jars were further maintained under laboratory condition (7 ± 3°C, 60 ± 10% RH,12L,12D) until adult emergence and effect of treatments on the F1 progeny were assessed. To avoid overlapping generation, the number of F1 progeny was counted upon emergence for a period of 45 days since the initial date of adult introduction.
Percentage reduction in adult emergence or inhibition rate (% IR) was calculated using the following formula.
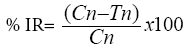
Where Cn=number of newly emerged insects in the untreated (control) jar; Tn=number of insects in the treated jar.
Grain damage assessment
Samples of 100 grains were taken randomly from the treated and control jars. Both treated and untreated grains were assessed for extent of bruchids damage using exit-holes as a measure of damage to the grain. The number of damaged grains (with characteristic hole) and undamaged grains were counted and weighed. Percentage grains weight loss was calculated using the following formula.
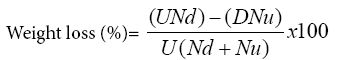
Where U=weight of undamaged grain, Nd=number of damaged grains; D=weight of damaged grain, Nu=number of undamaged grains.
Moreover, grains that are riddled with exit-holes were counted and the percentage damage (PD) and weevil perforation index (WPI) was calculated.
PD=(Total number of treated grains perforated/Total number of grains) × 100
WPI=(% of treated grains perforated/% of control grains perforated+% of treated grains perforated) × 100
Germination test
Germination test was carried out by randomly picking 80 undamaged grains from each treatment jar. Then 20 grains from each treated and control group were placed separately on a moistened filter paper (Whatman No.1) in Petri dishes and kept at room temperature. Each treatment was replicated four times where healthy grains without botanical insecticide powder application were used as a control. The numbers of germinated grains were recorded starting from the first date of germination. Percent germination was computed using the following formula [20].
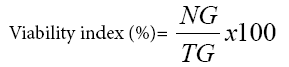
Where NG=number of grains germinated and TG=total number of grains tested in each Petri dish.
All data were checked for normality before they were subjected to analysis. Data which lacked normality were transformed using appropriate transformations method. Data were analyzed with analysis of variance (ANOVA) using General Linear Model (GLM) in SAS software (SAS Institute 2002-2008). Significant means were separated using Student-Newma Keuls (SNK) test.
Effect of different insecticide plants powder combination against A. obtectus mortality
The current study focused on adult mortality of Acanthoscelidesobtectusdue to differentplant powder treatments application about durations (24 hrs-96 hrs)on bean grain and treatments are undertaken in binary formulations (1%w/w or 2%w/w) shown in Tables 2 and 3. Typically best combinations of botanical powders had been selected for significantly increased (p<0.001) percentage adult mortality. There were binary formulations can have caused high mean mortality of A.obtectusafter the treatments had applied on bean grain for both binary formulations (1%w/w and 2%w/w).High mean mortality of adult bruchids had recorded in high dosage rate of insecticide plants powder (2%w/w) applications because of binary formulations. For instance, the binary formulations at 1%w/w were the cause of 66.42% A.obtectusmortality and increased 92.83% at 2%w/w after 96hrs duration of application. Over all, an increased mean adult insect’s mortality was recorded for both dosage rates of binary formulations as exposed long period of time (96hrs).
| Treatments | Adult Acanthoscelides obtectus mortality (% mean±SE) | |||
|---|---|---|---|---|
| 24hrs* | 48hrs | 72hrs | 96hrs | |
| E.globulus+A.sativum | 26.83±5.51c** | 38.33±9.08d | 50.83±9.08c | 66.42±2.08c |
| E.globulus+C. aurantifolia | 22.08±9.08d | 33.67±7.51d | 40.0±7.22d | 52.83±4.16e |
| E.globulus+E. tirucolli | 15.5±2.20e | 30.25±1.25e | 35.75±0.12d | 40.92±2.08h |
| E.globulus+J.curcas | 25.25±3.61c | 40.92±4.17d | 49.5±3.61c | 70.08±4.17c |
| E.globulus+V. amygdolina | 10.42±2.08e | 20.83±5.51e | 35.42±2.08d | 47.92±4.17e |
| A.sativum+C. aurantifolia | 34.17±7.51b | 55.25±9.55b | 66.83±9.08b | 74.92±7.51c |
| A.sativum+E. tirucolli | 28.5±3.61c | 42.58±2.08d | 53.08±4.17c | 63.5±3.61d |
| A.sativum+J.curcas | 39.92±2.08b | 60.75±3.10b | 70.92±2.08b | 88.67±7.51b |
| A.sativum+V. amygdolina | 27±3.61d | 47.75±3.61c | 56.42±7.51c | 75.08±9.08c |
| C. aurantifolia+E. tirucolli | 20.08±4.17d | 38.25±3.61d | 45.92±2.08c | 60.42±2.08d |
| C. aurantifolia+J.curcas | 28.5±3.61c | 49.83±4.17c | 57.25±6.25c | 68.33±4.17c |
| C. aurantifolia+V. amygdolina | 20.41±5.51d | 31.25±0.0b | 41.00±2.08d | 52.5±2.06e |
| E. tirucolli+J.curcas | 32.83±2.08b | 41.38±2.08d | 50.0±3.01c | 58.33±2.08d |
| E. tirucolli+V. amygdolina | 14.58±4.17e | 22.17±9.08e | 46.25±3.61d | 50.83±2.08e |
| J. curcas+V. amygdolina | 26.83±4.17c | 39.48±7.51d | 56.35±6.25c | 67.17±7.51c |
| Primiphos methyl | 90.65±7.22 a | 93.77±4.17a | 100±0.0a | 100±0.0a |
| Control (untreated) | 0.0±0.0h | 0.0±0.0g | 0.0±0.0k | 0.0±0.0I |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
*Hours after treatment application; **Means the same letter (s) within a column are not significantly different using Student-Newman-Keuls (SNK) test (P<0.05); ***Means 0.5 gram+0.5 gram of two different plant powder mixed with 100 g bean sample.
Table 2: Adult mortality (% mean ± SE) of Acanthoscelides obtectus on common bean seeds mixed with binary formulations (1%w/w) *** of different plants powder.
| Treatments | Adult Acanthoscelides obtectus mortality (% mean±SE) | |||
|---|---|---|---|---|
| 24hrs* | 48hrs | 72hrs | 96hrs | |
| E.globulus+A.sativum | 32.67±2.08e** | 57.92±4.17C | 81.58±2.51C | 92.83±4.17F |
| E.globulus+C. aurantifolia | 20.08±4.17d | 63.12±2.08B | 76.92±2.08B | 89.5±4.61C |
| E.globulus+E. tirucolli | 18.17±1.38g | 25.08±1.08E | 51±3.61E | 54.58±3.0H |
| E.globulus+J.curcas | 38.92±5.51d | 57.92±5.51C | 72.75±7.2C | 90.17±7.51F |
| E.globulus+V. amygdolina | 16.58±2.08F | 45.42±2.51D | 54.17±2.17D | 62.5±0.00I |
| A.sativum+C. aurantifolia | 37.33±2.08C | 60.42±4.17B | 73.83±2.08C | 85.25±0.00E |
| A.sativum+E. tirucolli | 29.17±4.17D | 59.25±3.61B | 67.5±3.61C | 72.92±5.51F |
| A.sativum+J.curcas | 45.25±3.61D | 67.17±5.51C | 78.83±2.08C | 93.25±3.61E |
| A.sativum+V. amygdolina | 35.33±2.08C | 66.42±8.33B | 72.92±5.51B | 85.33±2.08D |
| C. aurantifolia+E. trucolli | 31.42±4.17C | 54.58±2.08B | 62.0±3.61B | 80.58±2.08B |
| C. aurantifolia+J.cucas | 31.92±2.08D | 53.92±2.08C | 68.0±3.61B | 79.5±3.61C |
| C. aurantifolia+V. amygdolina | 25.42±5.51C | 39.33±2.08B | 43.83±5.51C | 60.42±5.51C |
| E. tirucolli+J.curcas | 35.25±3.61D | 48.92±5.51C | 70.75±6.25C | 78.08±2.08F |
| E. tirucolli+V. amygdolina | 18.5±3.61B | 28.17±2.08C | 45.42±5.51C | 67.67±5.51G |
| J. curcas+V.amygdolina | 30.92±4.17D | 49.92±4.17C | 66.58±5.51C | 70.83±8.33F |
| Primiphos-methyl | 87.5±7.22A | 91.67±4.17A | 100±0.0A | 100±0.0A |
| Control (untreated) | 0.00±0.00H | 0.00±0.00F | 0.00±0.00F | 0.00±0.00J |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
*Hours after treatment application; **Means the same letter (s) within a column are not significantly different using Student- Newman-Keuls (SNK) test (P<0.05); ***Means 1 gram+1 gram of two different plant powder mixed with 100g bean sample
Table 3: Adult mortality (% mean ± SE) of Acanthoscelides obtectus on common bean seeds mixed with unitary and binary formulations (2%w/w) *** of different plants powder.
High mean adult mortality for the combinations of A. sativum+J. curcas(88.67%) at 1%w/w and (93.25%) at 2%w/w had estimated after 96hrs treatment applications on bean grains.In this study each treatment combined with A. sativum or J.curcashad high potency compared to other binary formulations. For instance, lowest adult mortality due to E.globulus+E.trucolli(40.92%) at 1%w/w was recorded during long duration exposure, but in the same dosage rate of binary formulations and treatment application duration.E.globulus andE.trucolliincreased the potency combined withA. sativumto 66.42%, 63.5% respectively. comparatively no significance difference in mean adult mortality between (A. sativum+J. curcas) at 2%w/wand positive control (primiphose methyl) was proofed (96hrs).
Generally, in the present study showed (Tables 2 and 3) that high significance difference (p<0.001) of A.obtectusmortality between all treatments (1%w/w and 2%w/w) and untreated control after treatment applications.
Effects of botanical insecticides on percent F1 progeny production, Weevil Perforation Index (WPI) and percent grains weight loss
Mean of Weevil perforation index, percent F1 progeny produced and mean of inhibition were showed in Tables 4 and 5 for the application of plant powders binary formulations (1%w/w and 2%w/w) in bean grain. In current study, botanicals that scored high mean adult mortality had direct correlation with in decreasing weevil perforation index and weight loss as per both dosages of binary formulations. Some plant powder combinations showed (Table 4) high significant difference (p<0.001) in decreasing weevil perforation index because of high dosage binary formulation. For instance, A.sativum+J.curcashad low weevil perforation index (1.07%) comparison with J. curcas+V. amygdolina(19.61%) at the same dosage rate 2%w/w. Even these plant powders binary formulations had almost similar effect on decreasing percent weight loss, weevil perforation index by 1% w/w amount pace. In addition, increasing percentage of inhibition rate for the development F1 progeny and decreasing eggs formations were showed in Tables 4 and 5 because of both dosage rates of treatments. Moreover, there was no significance difference among treatments and lowest emerging of F1 progenies was recorded due to 2%w/w. For example, E.globulus+J.curcas(0.53%), A.sativum+J.curcas(0.03%),A.sativum+V.amygdolina(0.13%),andC. aurantifolia+E. tirucalli(0.67%)had high mean against emerging ofF1 progeny. Some treatments those had high mean inhibition rate or percentage reduction in adult emergence records were no significance difference as compared to Primiphos-methyl (positive control), at 2%w/w binary formulations.
| Treatments | F1 progeny | % (IR) | (WPI*) | WL |
|---|---|---|---|---|
| E.globulus+A.sativum | 4.3±0.18c** | 90.12±2.56a | 9.87±4.53b | 0.33±0.15c |
| E.globulus+C. aurantifolia | 8.00±1.0c | 78.51±1.07b | 15.61±4.51b | 0.64±0.04c |
| E.globulus+E. tirucalli | 14.03±3.18e | 59.52±13.31c | 40.10±1.26c | 1.60±0.50b |
| E.globulus+J.curcas | 1.00±2.08d | 97.32±5.29a | 2.69±0.02f | 0.03±0.26c |
| E.globulus+V. amygdolina | 18.00±1.73e | 52.03±4.13c | 42.58±2.50c | 1.29±0.18b |
| A.sativum+C. aurantifolia | 1.30±1.23d | 93.21±3.09a | 10.20±3.24b | 0.05±0.15c |
| A.sativum+E. tirucalli | 5.22±2.19c | 85.25±5.10b | 13.10±0.56b | 0.19±0.03c |
| A.sativum+J.curcas | 0.83±0.30d | 98.19±0.20a | 2.85±2.58f | 0.08±0.12c |
| A.sativum+V. amygdolina | 3.33±0.84c | 91.00±0.38a | 11.00±3.49b | 0.26±0.31c |
| C. aurantifolia+E. tirucalli | 4.83±0.88c | 87.59±3.15a | 12.14±2.68b | 0.30±0.60c |
| C. aurantifolia+J.curcas | 2.67±0.33c | 96.08±0.19a | 3.46±2.53f | 0.09±0.00c |
| C. aurantifolia+V. amygdolina | 9.00±1.20c | 73.55±5.00b | 22.00±0.23b | 1.21±0.50b |
| E. tirucalli+J.curcas | 3.67±1.20c | 80.55±3.25b | 20.63±1.94b | 1.18±0.09b |
| E. tirucalli+V. amygdolina | 4.00±1.15c | 80.59±1.39b | 17.17±2.63b | 1.25±0.13b |
| J. curcas+V. amygdolina | 11.55±1.20e | 65.57±4.03c | 33.02±0.84c | 1.52±0.09b |
| Primiphos-methyl | 0.01±0.01g | 99.76±0.24a | 0.86±1.19d | 0.01±0.04c |
| Control (untreated) | 62.00±7.00a | 0.00±0.00d | 55.00±0.00a | 2.12±0.61a |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
*WPI>50 indicate negative protectant ability; **Means followed by the same letter within a column are not significantly different using Student-Newman-Keuls (SNK) test (P<0.05); ***Means 0.5 gram+0.5 gram of two different plant powder mixed with 100 g bean sample
Table 4: Mean of F1 progeny produced (mean ± SE), % inhibition rate (IR), weevil perforation index (WPI) and % weight loss caused by Acanthoscelides obtectus on common bean seeds mixed with binary formulation different plants powder at (1%w/w) *** dosage rate.
| Treatments | F1 progeny | (%IR) | (WPI*) | %WL |
|---|---|---|---|---|
| E.globulus+A.sativum | 2.00±2.01c** | 95.96±2.86a | 2.35±2.53d | 0.13±0.18b |
| E.globulus+C. aurantifolia | 4.33±0.13c | 85.26±0.64c | 11.42±2.20f | 0.20±0.07b |
| E.globulus+E. tirucalli | 7.33±2.23c | 75.11±2.00d | 18.06±1.31b | 0.44±0.05b |
| E.globulus+J.curcas | 0.53±5.03c | 98.78±4.25a | 1.29±5.79c | 0.07±0.17b |
| E.globulus+V. amygdolina | 9.33±1.26c | 69.59±5.19d | 25.41±1.79b | 0.57±0.31b |
| A.sativum+C. aurantifolia | 0.63±2.10c | 98.17±0.57a | 1.09±2.37c | 0.03±0.09b |
| A.sativum+E. tirucalli | 1.98±0.88c | 96.96±0.53a | 2.35±1.95c | 0.16±0.17b |
| A.sativum+J.curcas | 0.03±0.01c | 99.02±0.20a | 1.07±2.26c | 0.03±0.03b |
| A.sativum+V. amygdolina | 0.13±3.35c | 97.76±0.91a | 1.78±2.31c | 0.10±0.09b |
| C. aurantifolia+E. tirucalli | 0.67±0.33c | 98.30±0.17a | 2.14±1.09c | 0.14±0.04b |
| C. aurantifolia+J.curcas | 0.50±0.20c | 98.00±0.21a | 1.11±1.50c | 0.03±0.00b |
| C. aurantifolia+V. amygdolina | 8.00±0.30c | 81.10±2.32c | 17.00±1.70b | 0.40±0.00b |
| E. itrucalli+J.curcas | 7.00±1.10c | 84.44±2.94c | 14.94±5.05b | 0.28±0.09b |
| E. tirucalli+V. amygdolina | 6.33±0.48c | 87.48±1.19a | 10.07±2.83f | 0.15±0.23b |
| J. curcas+V. amygdolina | 7.33±0.13c | 79.56±5.75c | 19.61±2.71b | 0.49±0.22b |
| Primiphos methyl | 0.01±0.33c | 99.26±0.54a | 0.79±1.19e | 0.01±0.04b |
| Control (untreated) | 50.00±4.10a | 0.00±0.00b | 48.00±0.00a | 2.51±0.41a |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
*WPI value above 50 indicate negative protectant ability; **Means followed by the same letter within a column are not significantly different using Student-Newman-Keuls (SNK) test (P<0.05); ***Means 1 gram+1 gram of two different plant powder mixed with 100 g bean sample; WPR=Weevil Perforation index, IR=Inhibition Rate, WL=Weight loss
Table 5: Mean of F1 progeny produced (mean ± SE), % inhibition rate (IR), weevil perforation index (WPI) and % weight loss caused by Acanthoscelides obtectus on common bean seeds mixed with binary formulation different plants powder at (2%w/w) *** dosage rate.
Generally, the present-day study showed (Tables 4 and 5) that all treatments had effect of decreasing the emergence of F1 progeny, increasing percentage inhibition rate and decreasing percentage weight loss as well as weevil perforate index while compared with control (untreated). However, majority of treatments in bean grains showed best toxicity against whatever a matter of A. obtectus, there was the lowest potency recorded in binary formulation of (E.globulus+E. tirucalli) at both dosage rates.
Effects of binary botanical formulations treatment on percent germination
Germination percent of common bean seeds treated with different binary botanical powder formulation is presented in Table 6. There was no significant (P>0.05) difference in the percent germination between disinfected common bean seeds treated with different botanical insecticide formulations and untreated control at both dosage rates. The percent germination of bean seed treated with different botanical powder formulations ranged between 92-99%, which was as good as untreated control, indicating botanical treatment didn’t have effect on germination rate.
| Treatments | Percent Germination (mean ± SE) | |
|---|---|---|
| 1%w/w | 2%w/w | |
| E. globulus+A. sativum | 98.17 ± 1.12a* | 98.00 ± 0.36a |
| E. globulus+C. aurantifolia | 97.65 ± 0.65a | 96.52 ± 0.16a |
| E. globulus+E. tirucalli | 94.08 ± 1.24a | 99.50 ± 0.15a |
| E. globulus+J. curcas | 95.42 ± 1.24a | 98.92 ± 0.74a |
| E. globulus+V. amygdolina | 99.75 ± 0.15a | 97.28 ± 1.55a |
| A. sativum+C. aurantifolia | 94.67 ± 2.94a | 96.17 ± 1.65a |
| A. sativum+E. tirucalli | 95.00 ± 1.51a | 96.27 ± 1.07a |
| A. sativum+J. curcas | 92.50 ± 1.90a | 98.08 ± 1.09a |
| A. sativum+V. amygdolina | 98.12 ± 0.24a | 96.67 ± 1.12a |
| C. aurantifolia+E. tirucalli | 99.82 ± 0.19a | 96.28 ± 3.12a |
| C. aurantifolia+J. curcas | 98.30 ± 0.54a | 99.17 ± 0.56a |
| C. aurantifolia+V. amygdolina | 97.65 ± 1.02a | 98.50 ± 0.54a |
| E. itrucalli+J. curcas | 96.58 ± 1.78a | 97.23 ± 0.74a |
| E. tirucalli+V. amygdolina | 96.42 ± 1.34a | 97.12 ± 2.24a |
| J. curcas+V. amygdolina | 93.33 ± 1.88a | 94.03 ± 2.92a |
| Primiphos methyl | 98.03 ± 0.31a | 98.03 ± 0.63a |
| Control (untreated) | 97.22 ± 0.76a | 98.94 ± 0.56a |
| P-value | P=0.5308 | P=0.7992 |
Table 6: Effect of binary botanical formulations treatment on percent germination (mean ± SE) of common bean seeds.
The previous studies have been attempted to get a solution for protection of crop pests by using botanicals (alternative method) ingredients or powders. Although, many studies have tested several botanicals individually as an alternative protection of insects and pathogenic microorganisms, only a few studies have considered botanicals combination. The current study aimed on to evaluate the combined effect of insecticide botanicals powder against bruchids (A. obtectus) which usually damage economically important crop (common bean) before and after harvesting. In the present study significantly, different mean A. obtectusmortality was recorded with respect to exposure time of treated bean and variety of binary formulations.
High mean adult insect mortality was recorded during the last exposure time (96hrs) of treated bean at both dosage rates (1%w/w and 2%w/w), conversely, there was low adult A. obtectusmortality record to the first day (24hrs) of exposure duration.For instance, the binary formulation (A. sativum+V.amygdolina) of mean insect mortality was 27% with the first day (24hrs) and this was increased to 75.08% after four days (96hrs) of treatment application at lower dosage. Also, the current study accords with the previous finding done by GetuE (2009). Long duration exposure of treated bean that contain adult insects had high percent mortality as compared with short exposure. the variety in adult toxicity of such botanicals were probably due to difference in the types and levels of active ingredients that depend on not only the genetic characteristics of the plant species, but also the conditions under which they were grown and harvested [21].
Some studies have tried to investigate the potency of insecticide botanicals combination. Combining more than two botanicals would be challenging for pests to exhibit resistance [9,22]. In this study any individual treatment that combined with A. sativumhas increased its toxicity when compared with other combinations at both dosage rates. For example, significantly high (p<0.0001) mean bruchids (A. obtectus) mortality was recorded due to treatments combined with A sativum. Such as, (V.amygdolina+E. globules) caused 47.92% mean of insects’ mortality and when V.amygdolinacombined with A sativum, the potencyhas augmented to 75.08% at low dosage rate after four days (96hrs) treatment applications. The current study in agreement with study investigated by Oparaeke and Dike. An investigation was conducted to compare the products of A. sativum and C. citratusin the control of C. maculateson stored cowpea grains and They found that both plant powders showed effectiveness by exhibiting 100% mortality 7 days after treatment. In addition, Danjummaand co. also found that A. sativum is effective in killing adult S.zeamaisand recorded 96.67% mortality at the rate of 2.0 g/50 g maize grains [23]. The mode of action of these two plant powders may be due fumigant and anti-feedant effects. A. sativumpowder contains allicin as the major constituent.
Furthermore, significantly high insects’ mortality was recorded due to all treatments compared to untreated (control). Moreover, each treatment of insecticide plants revealed that the reduction of F1 progeny production, reduction of weevil perforate index and increased inhibition rate against Acanthoscelidesobtectusat both dosage rates of binary formulations while compared to untreated bean grains. Particularly, some combined treatments of current study had high percentage of inhibition rate or percentage reduction in the adult emergence, such as,E.globulus+J.curcas(98.78%), A.sativum+C. aurantifolia(98.17%),A.sativum+J.curcas(99.02%), at 2%w/w dosage rate and these treatments had no significant difference in percentage reduction in the adult emergence comparison withPrimiphos-methyl. There was direct relationship between treatments that had high mean adult mortality record and high inhibition rate i.e., treatments which had high mean adult mortality as well as they had high inhibition rate, low weevil perforate index and weight loss. For instance,A.sativum+J.curcas, and E.globulus+J.curcastreatments had high mean score in adult mortality due to both dosage rates, otherwise these formulations had high inhibition rate low weevil perforate index at both dosage rates and this was true for other binary formulations.on the other hand, all botanicals powder had no significant difference to produce F1 progeny by A. obtectusand each insecticide plant powders significantly reduced the emergence of F1 progeny as compared to untreated (control) bean grains, like wise no significant difference to all treatments for the lessening of bean grain weight loss at high dosage rate binary formulation of treatments.
The previous study investigated that if the plant powders reduce adult longevity and fitness, the number of eggs laid will often be lower as well. Moreover, the mechanical effect of large quantities of powders themselves could influence oviposition [24]. The current study agreement with the previous study investigated by Openshaw K thatJ. curcaswas a multipurpose plant with many properties and considerable insecticidal potential.Different parts of J. curcascontain the curcin and phorbol ester which are toxic alkaloids that inhibit animals from feeding on it [25,26]. The insecticidal and inhibition of progeny emergence activities of oil extracted from seeds of J. curcashas been reported by earlier researchers against several insect pests [27].
According to many authors, any substance that reduces food consumption by an insect can be considered as an antifeedant. For instance, Ismanet al.defined antifeedants as behavior-modifying substances that deter through a direct action on taste organs in insects [28]. This definition excludes chemicals that suppress feeding by acting on the central nervous system, or a substance that has sublethal toxicity to the insect. Feeding inhibition in insect pests is the most important in the search for new and safer methods for pest control in stored grains.
Related studies showed botanical powder treatment act as oviposition-deterrent, inhibit oviposition by weakening adult bruchid to lay fewer eggs and kill the hatching larvae afterwards [17,29,30]. In related study, even though synergistic combination of botanicals in insect suppression has not been widely examined, several studies revealed the potential of botanical insecticides in reducing F1 progeny production on different insect [14,17,31].
Generally, the current study showed that treatments that had high dosage rate binary formulations results better efficacy with respect to increased adult mortality, decreasing F1 progeny emergence, decreasing weevil perforate index and weight loss. All treatments of the current study caused high mean mortality of adult insect (A. obtectus) when compared with the normal/ control bean grains after 96hrs treatments application. Some binary formulations of botanicals par with Primiphos-methylregard to toxicity against adult insect mortality and decreasing F1 progeny emergence. Additionally, these treatments had equivalent efficacy with Primiphos-methylin decreasing both weevil perforate index and weight loss of treated bean grains. For instance, E.globulus+J.curcas, A.sativum+J.curcas, and C. aurantifolia+J.curcasat low dosage rate (1%w/w) treatment application.High dosage of these treatments had no significant difference with positive control (Primiphos-methyl) in decreasing both weevil perforate index and weight loss of treated bean grains including reduction of F1 progeny.
The germination test of treated bean grains of current investigation did not affect i.e., all treatments had no effect on germinations of treated grains. The viability of seed is necessary for planting and food. There was no significance difference among treatments to the germination of treated bean grains. The current investigation agreement with previous finding reported by Dejen, Rahman and Talukder [17,32] botanicals that were toxic to pest (insects can damage grains) did not affect the viability of seeds after usage as protection of pests. Related investigation also reported that seeds treated with unitary botanical formulation showed no significant effect on the germination rate [20].
In summery high dosage rate (2%w/w) of treatments of current study had high mean insect mortality when compared with low dosage rate (1%w/w) after 96hrs treatment application. There was high mean insect mortality in long time exposure of treatments and this was accord withprevious study reported by Getu [20]. Long duration exposure of treated bean that contain adult insects had high percent mortality as compared with short exposure. The current study revealed that two botanical powders were highly effective to the mortality of insects as compared with the rest of treatments i.e., they had high mean adult insect mortality record such as,A. sativum and J.curcas.The combination of these botanicals had equivalent toxicity to the chemical powder (Primiphos-methyl). Individual treatment which combined with A.sativum and J.curcasincreased the trend of toxicity against the insect. The effectiveness of garlic in reducing aphid population can be attributed to the fact that the plant contains a group of closely related compounds (allicins) which are responsible for the pesticidal properties and repellence against aphids [33]. Aqueous extracts of J.curcas leaves were effective in controlling Sclerotium sp., an Azollafungal pathogen [34]. In laboratory experiments, ground J.curcas showedmolluscicidal activity against the host of liver fluke (Lymnaeaauriculariarubiginosa), a disease which is widely distributed in the Philippines and against the hosts of Fasciolagiganteaand Schistosomiain Senegal. Extracts from crushed whole seeds showed molluscicidal activity against several schistosome vector snails [35-37]. Phorbol esters were probably the active agents in the different extracts used.
The author recommended that botanical combinations should have to advertise to local farmers who had less income and the national government should have to encourage such like finding.
The author is grateful to Melkassa Research Center of Ethiopian Institute of Agricultural Research for providing necessary laboratory facilities for undertaking research and received sponsorship to undertake this research as part of his MSc study from Hawassa University. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.