Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2019)Volume 7, Issue 1
Objectives: A study was undertaken to isolate bacteria from fermented dairy products and evaluate their probiotic potential.
Methods: From the colonies formed on MRS agar plates, 8 bacterial isolates were selected and evaluated for their anti-microbial and cholesterol assimilation capability.
Results: Among the tested isolates, P3 showed maximum antimicrobial activity against Staphylococcus aureus ATCC 6538 and P8 showed maximum antimicrobial activity against Salmonella enteritidis ATCC 13076, Listeria monocytogenes ATCC 7644 and Escherichia coli ATCC 35150. The isolate P8 also showed highest cholesterol assimilation capability (41.91%) which is followed by isolate P3 (35.95%). Based on the morphological, biochemical and physiological characteristics the isolate P3 and P8 were identified as Lactococcus lactis and Streptococcus thermophilus, respectively.
Conclusion: The studied fermented dairy products available in Bangladesh contain adequate number of lactic acid bacteria and some of the bacterial isolates possess appreciable probiotic properties.
Probiotics; Streptococcus; Lactococcus; Dairy products
Probiotics are defined as microorganisms that are believed to provide health benefits when consumed [1]. These bacteria are considered as ‘Generally Recognized as Safe’ (GRAS) organisms and are used in the preparation of fermented food products [2]. Probiotic is usually a dairy food or a dietary supplement containing live bacteria that replace or add to the beneficial bacteria normally present in the gastrointestinal tract of man and animal.
Probiotic bacteria exert several health benefits to the consumer in several ways: Improvement of the health of intestinal tract, enchantment of the immune system, synthesis and enhancement of the bioavailability of nutrients, reduction of the lactose intolerance, decrease of the prevalence of allergy in susceptible individuals, and reduction of the risk of certain cancers [3].
The cholesterol-reducing effect of probiotic has become more apparent with the discovery of bile salt deconjugating and cholesterol assimilating ability of Lactobacillus [4,5]. Thereafter, a set of screening procedures both in vitro and in vivo was established for evaluation of cholesterol-reducing probiotics [6]. Many probiotic strains mostly L. acidophilus were screened out with cholesterol-reducing property [7].
In Bangladesh, different types of fermented dairy products are very popular among the people of all age groups. However, a few works have been done on the diversity of microbes present in those probiotic products and evaluation of the probiotic properties of individual bacteria present in those probiotic products. In this study, attempts were taken to isolate bacteria from the local fermented dairy products and evaluate their probiotic potential.
Collection of samples
Eight different fermented dairy products (curd, locally known as ‘doi’) were collected from different shops of Dhaka and Bogra Districts of Bangladesh. After collection, samples were transferred aseptically to Laboratory of Microbiology & Industrial Irradiation Division (MIID) of Institute of Food & Radiation Biology (IFRB), Atomic Energy Research Establishment (AERE), Ganakbari, Savar, Dhaka and immediately stored at 4°C for further analysis.
Isolation of bacteria
Isolation of Lactic acid bacteria (LAB) was done using the MRS (de Man, Rogosa and Sharpe) agar medium. One gram of sample was added to 9 mL of physiological saline (0.9% NaCl) and mixed well. This sample suspension is then serially diluted up to 105 times and 0.1 mL sample from each dilution was plated onto MRS agar medium 0.5% CaCO3 (pH 6.2 ± 0.2). The plates were incubated at 37°C for 24 h. The clear zone forming discrete colonies were counted and selected for further study.
Identification of the isolates
Selected isolates were subjected to different morphological, biochemical and physiological tests. Colony characteristics including colony size, shape, consistency, elevation etc. were recorded. Physiological tests like bile salt tolerance, NaCl tolerance, growth at different temperature and low pH tolerance and biochemical tests including catalase, oxidase, MR-VP, citrate utilization, bile esculine test, hemolytic activity and various sugar fermentation tests were performed.
Bile salt tolerance assay
Growth rate of bacterial cultures was determined in MRS Broth containing different levels of bile bovine, (Sigma, USA) (0.5, 1, 1.5 & 2%). Freshly prepared cultures were inoculated (1%) into medium and incubated at 37°C for 24 hours under anaerobic condition. Optical density of each sample was measured using a spectrophotometer at 560 nm against uninoculated broth (0%) [8].
pH tolerance assay
For pH tolerance assay, 100 μL of fresh culture was inoculated into 10 mL MRS broth with varying pH ranging from 2, 3, 4, 5, 6 and 8. The pH was adjusted with concentrated hydrochloric acid (HCl) and 0.1N sodium hydroxide (NaOH). The inoculated broth was incubated in anaerobic condition for 24 hours at 37°C. After incubation, growth of the bacteria was measured using a spectrophotometer at 560 nm against uninoculated control broth.
NaCl tolerance assay
For the determination of NaCl tolerance, each isolate was grown in MRS broth containing six test tubes that were adjusted the concentration of NaCl (0%, 1%, 2%, 3%, 4%, 5% and 6.5%) After autoclave, each test tube was inoculated with 100 μL overnight culture of the isolates and incubated anaerobically at 37°C for 24 hours. After incubation, the bacterial growth was measured using spectrophotometer at 600 nm against uninoculated control broth [9].
Assessment of antimicrobial activity
Antimicrobial activity is one of the most important selection criteria of probiotics. Agar-well diffusion assay was used to determine the antimicrobial activity of the isolated LAB [10]. Bacterial strains belonging to both gram-positive and gramnegative groups such as Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 35150 Salmonella enteritidis ATCC 13076, Listeria monocytogenes ATCC 7644 were used as test organism.
The LAB isolated from fermented dairy products were grown in MRS broth and the culture filtrate was collected by centrifugation of the culture. Then 30 μl supernatant was poured into the well (6 mm diameter) of Mueller Hinton Agar (MHA) containing test bacteria. The plates were incubated at 37°C for 24 hours. The zone of inhibition measured in nearest mm.
Cholesterol assimilation test
The human blood was collected from healthy individuals and centrifuged at 2500 rpm for 15 minutes. The supernatant was separated and the potential LAB isolates were separately inoculated in each tube except the control tube and anaerobically incubated for 24 hours at 37°C. After the incubation, the culture was centrifuged at 10000 rpm for 15 minute and supernatant was collected for measuring the cholesterol assimilation by the isolates following the method described by Rudel and Morris [11].
Morphological and cultural characteristics of the isolates
Eight bacterial isolates which formed clear zone on MRS Agar +0.5% CaCO3 medium and were non-motile and catalase negative were selected and purified for further study. The morphological and cultural properties of the isolates are presented in Table 1.
| Isolates | Cultural characteristics (MRS Agar) | ||||||
|---|---|---|---|---|---|---|---|
| Shape | Colony color | Colony size* | Colony shape | Consistency | Colony elevation | Appearance | |
| P1 | Round | Whitish | Medium | Circular | Sticky | Raised | Shiny |
| P2 | Round | Creamy | Small | Circular | Sticky | Raised | Shiny |
| P3 | Round | Whitish | Medium | Irregular | Sticky | Umbonate | Dull |
| P4 | Round | Whitish | Medium | Circular | Sticky | Raised | Shiny |
| P5 | Round | Whitish | Small | Circular | Sticky | Raised | Shiny |
| P6 | Round | Whitish | Small | Circular | Sticky | Raised | Shiny |
| P7 | Round | Whitish | Small | Circular | Sticky | Raised | Shiny |
| P8 | Round | Whitish | Medium | Circular | Sticky | Umbonate | Shiny |
Table 1: Morphological and cultural characteristics of the isolated bacteria. *Colony diameter less than 0.5 mm denoted as pointed, 0.5-1.0 mm denoted as small, 1.0-2.0 mm denoted as medium and more than 2.0 mm denoted as large colony.
Physiological and biochemical characterization
Bile salt tolerance assay: The bile salt tolerance capacity of the isolates (P3 and P8) is shown in Figure 1. Both the isolates were able to grow well in presence of 2% bile salt. The isolate P3 showed best bile salt tolerance.
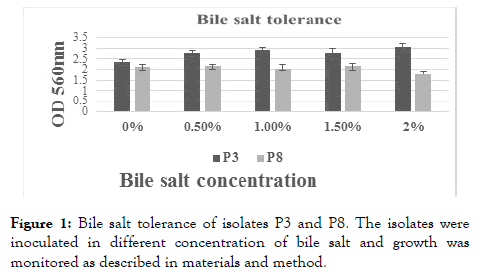
Figure 1. Bile salt tolerance of isolates P3 and P8. The isolates were inoculated in different concentration of bile salt and growth was monitored as described in materials and method.
pH tolerance assay
The pH tolerance capacity of the isolates (P3 and P8) is shown in Figure 2. The best growth of the isolates was observed at pH 6.5. However, moderate growths of both the isolates were observed at pH 3.0.
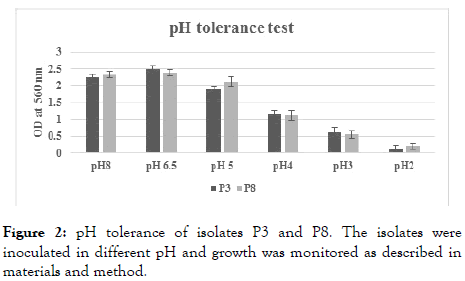
Figure 2. pH tolerance of isolates P3 and P8. The isolates were inoculated in different pH and growth was monitored as described in materials and method.
Biochemical characterization
Various biochemical tests were performed for the identification of isolate P3 and P8 are presented in the Table 2. Based on the cultural, morphological and biochemical characteristics, isolate P3 was identified as Lactococcus lactis and the isolate P8 was identified as Streptococcus thermophilus.
| Test | P3 | P8 | |
|---|---|---|---|
| Gram Staining | + | + | |
| Growth at 10°C* | + | + | |
| Growth at 45°C* | ++ | ++ | |
| Growth at pH 9.6 | - | - | |
| Growth in 6.5% NaCl | - | - | |
| Catalase test | - | - | |
| Bile Esculine test | - | - | |
| Methyl Red test | + | - | |
| VP test | + | + | |
| pH after 48 hours in MRS media | 3.8 | 3.7 | |
| Citrate utilization test | - | - | |
| Hemolytic activity | ᵧ | ᵧ | |
| Carbohydrate fermentation test | Glucose | + | + |
| Sucrose | + | + | |
| Maltose | - | - | |
| Mannose | + | + | |
| Trehalose | + | - | |
| Sorbitol | - | - | |
| Lactose | + | + | |
| Galactose | - | - | |
| Arabinose | + | + | |
| Ribose | + | - | |
| Starch | - | - | |
| Mannitol | - | - | |
| Xylose | - | - | |
| Provisionally identified as | Lactococcus lactis | Streptococcus thermophilus | |
Table 2: Biochemical and physiological characteristics of the isolates. *+=Poor growth and ++=moderate growth.
Antimicrobial activity
The antimicrobial activity of the culture supernatant of the isolate P3 and P8 were tested against four pathogenic microorganisms in MHA media. P3 showed maximum activity against Staphylococcus aureus ATCC 6538 and P8 showed maximum activity against Salmonella enteritidis ATCC 13076 (Table 3).
| Test organisms | Zone of inhibition (mm) | |
|---|---|---|
| P3 | P8 | |
| Escherichia coli ATCC 35150 | 11.4 | 15.1 |
| Staphylococcus aureus ATCC 6538 | 15 | 13.3 |
| Salmonella enteritidis ATCC 13076 | 13.9 | 15.4 |
| Listeria monocytogenes ATCC 7644 | 13.1 | 14.4 |
Table 3: Zone of inhibition (mm) produced by different isolates against test organisms.
Cholesterol assimilation capacity
In this test, the serum cholesterol taken for analysis of cholesterol assimilation capacity of the isolates. The sample P3 and P8 showed 35.95% and 41.92% cholesterol assimilation capability respectively (Figure 3).
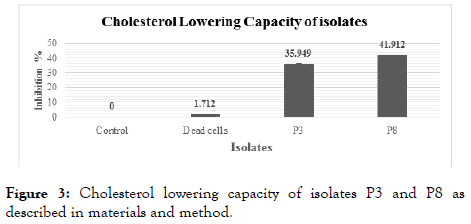
Figure 3. Cholesterol lowering capacity of isolates P3 and P8 as described in materials and method.
In this study attempt was taken to isolate LAB from dairy products and assess their anti-bacterial activity and the ability to assimilate cholesterol. The randomly selected 8 isolates were further purified and the morphological, cultural and biochemical properties of two isolates (P3 and P8) were studied. According to Xanthopoulos et al. [12] the ability to tolerate bile salts varies a lot among the LAB species and between strains themselves. Bile resistance of some isolates is related to the activity of bile salt hydrolase (BSH) that helps to hydrolyze conjugated bile, reducing its toxic effect [13]. BSH activity has most often been found in microorganisms isolated from the intestines or feces of animals [14]. The capacity to survive in the gastrointestinal tract by tolerating and colonizing at low pH in stomach (up to pH 3), higher pH in small intestine (like pH 8) and higher bile salt (up to 2%), are important features of probiotics. 8 isolates showed low pH tolerance and survival in the presence of bile salt even after 24 h of incubation. Liong and Shah [15] reported survival of LAB in the presence of high concentration of bile salt. The finding of this research also corroborates the findings of Guo et al. [16] where LAB isolates survived the presence of 0.3% Oxgall.
Isolates (P3 and P8) showed antibacterial activity against four bacterial strains which fulfills another criterion to be used as those isolates as probiotics. Through this finding it may be concluded that the isolated LAB may be used to prevent foodborne bacteria and improve the microflora of gastro-intestinal tract [17].
The use of probiotics in reducing serum cholesterol levels has attracted much attention since blood cholesterol reduction through dietary modification is preferred over drugs. Isolate P3 and P8 showed significant assimilation of cholesterol. Isolate P3 assimilated 41.92% cholesterol. This ability is higher than previously reported L. lactis subsp. Lactis LQ. The cholesterol assimilation capable isolates may be used as oral supplement which may prevent the hypercholesterolemia and reduce the coronary heart disease as well. Although findings of this study in appreciable but it will be wise to use these isolates as probiotic after double-blind trial using human subjects.
Citation: Rahman MR, Kabir MS, Khan ZUM, Pramanik MK (2019) Assessment of Probiotic Properties of Some Bacteria Isolated from Dairy Products. J Prob Health. 7:207. doi: 10.35248/2329-8901.19.7.207
Received: 03-Jan-2019 Accepted: 18-Jan-2019 Published: 25-Jan-2019 , DOI: 10.35248/2329-8901.19.7.207
Copyright: © 2019 Rahman MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.