Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2016) Volume 7, Issue 4
The viscosities (η) and densities (ρ) have been measured for the binary mixtures of dimethyl carbonate (DMC) with 2-alkoxyethanols such as 2-methoxyethanol (MOE), 2-ethoxyethanols (EOE) and 2-butoxyethanols (BOE) over the entire range of mole fraction at T=(303.15, 308.15, 313.15, 318.15) K and at constant atmospheric pressure. The excess/deviation properties such as deviation in viscosity and excess Gibbs free energy of activation of viscous flow are calculated. Excess/deviation properties are correlated by the Redlich-Kister equation to obtain the binary coefficients and standard deviations. Further several semi empirical models such as Grunberg-Nissan, Katti- Chaudhri, Heric-Brewer and Hind et al. are used to correlate the viscosity of binary mixtures. The values of Δη, which refers to the deviation of the experimental values of the viscosity of the mixture from the mole fraction mixture law rules, are found to be negative for all the mixtures. The results are discussed in terms of molecular interactions due to physical, chemical and structural effects between the unlike molecules.
Keywords: Viscosity, Gibbs free energy, Redlich-Kister equation, Molecular interaction
Viscosity and density data for binary liquids are important from practical and theoretical point of view. Experimental measurements of these properties of binary mixtures have gained much importance in many chemical industries and engineering disciplines [1]. Knowledge of the viscosity is very important in many chemical applications, such as mass and heat transfer operations, fluid flow, molecular structure and design involving chemical separations, developing separation methods like HPLC and capillary electrophoresis etc. Dimethyl carbonate (DMC) is considered to be a green solvent. It is a nontoxic substance and is widely used as a replacement for dimethyl sulphate, methyl halide, and phosgene in methylation and carbonylation reactions, because it is considered to be an “environmentally benign building block” [2]. Dialkyl carbonates have shown to be very useful in the lithium battery technology [3,4]. DMC has about 3 times the oxygen content as methyl tert-butyl ether (MTBE) and it is a strong contender to assist the refining industry. It does not phase separate in a water stream as some alcohols do, and it has both low toxicity and relatively quick biodegradability [5,6]. Glycol ethers are a group of solvents based on alkyl ethers of ethylene glycol or propylene glycol commonly used in paints and cleaners. Among cellosolves i.e., Alkoxyethanols viz. 2-methoxyethanol (MOE), 2-ethoxyethanol (EOE), 2-butoxyethanol (BOE) as oxygenated compounds are increasingly used as additives to gasoline due to their octane enhancing and pollution-reducing properties [7,8]. One of the interesting features of the chemicals that are selected in this study, are used as green solvents in gasoline industry.
In the present paper, we report viscosity, deviation in viscosity and excess Gibbs free energy of activation of viscous flow data for the binary mixtures of 2-methoxyethanol, 2-ethoxyethanol, 2-butoxyethanol with dimethyl carbonate at four different temperatures T=(303.15, 308.15, 308.15, 313.15) K. These Excess/deviation properties are correlated by the Redlich-Kister equation to obtain their binary coefficients and standard deviations. This work will also provide a test of various semi empirical relations like Grunberg-Nissan, Katti- Chaudhri, Heric-Brewer and Hind et al. to correlate viscosity of binary mixtures. Literature about binary liquid with one of the solvent as dimethyl carbonate is plenty [9-14]. A deep literature survey reveals that no significant work is available on the binary mixtures of dimethyl carbonate and 2-alkoxyethanols at a temperature range of (303.15- 318.15) K.
DMC was obtained from Aldrich Chemical Co., stated purity 99 mol%. The chemicals 2-methoxyethanol, 2-ethoxyethanol and 2-butoxyethanol are obtained from SD Fine Chemicals Ltd., India, stated mass fraction purity>0.995 are used in this study. Before measurements, all the liquids were kept in dark bottles, dried over molecular sieves (Union Carbide, type 4A), and degassed it ultrasonically. All the chemicals were purified by method described in literature [15,16]. The chemicals after purification were 99.8% pure and their purity was ascertained by GLC and also by comparing experimental values of density and viscosity, at 303.15 K with those reported in the literature, as presented in Table 1.
| Compound | ρ (10−3 kg m-3) | η (m Pa s) | ||
|---|---|---|---|---|
| Expt. | Lit. | Expt. | Lit. | |
| DMc | 1.0567 | 1.056719a | 0.549 | 0.549b |
| MOE | 0.9556 | 0.95572c | 1.422 | 1.4212c |
| EOE | 0.9212 | 0.92119c | 1.655 | 1.6541c |
| BOE | 0.8922 | 0.89228d | 2.477 | 2.404e |
a-[17], b-[18], c-[19], d-[20], e-[21]
Table 1: Comparison of density (ρ) and viscosity (η) of the pure liquids with literature data at 303.15°K.
The binary mixtures are prepared gravimetrically using an electronic balance (Shimadzu AY120) with an uncertainty of ± 1 × 10−7 kg and stored in airtight bottles. The uncertainty on mole fraction is estimated to be 1 × 10−4. It is ensured that the mixtures are properly mixed and the measurement of the required parameters was done within one day of preparation.
The viscosity, η, of the pure liquids and liquid mixtures is determined using an Ubbelohde suspended-level viscometer. The viscometer is suspended in a thermostatic water bath in which the temperature is maintained constant to ± 0.01 K. Five sets of readings for the flow time are taken by using a Racer stop watch that can register time to ± 0.01 s, and the arithmetic mean is taken for the calculation of the viscosity. Because the flow times are greater than 200 s and the capillary diameter is 0.55 mm, which is much less than the tube length of 100 mm, both kinetic energy and tube end corrections are negligible. At each temperature, the viscometer was calibrated against the known viscosities of benzene and carbon tetrachloride [22]. Viscosity of pure liquids and liquid mixtures were calculated using the following relation:
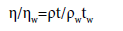 (1)
(1)
The estimated uncertainty in the viscosity measurements is found to be less than 1%.
The densities (ρ) of pure liquids and their mixtures are determined using a 10−5 m3 double-arm pycnometer, and the values from triplicate replication at each temperature are reproducible within 2 × 10–1 kgm-3. The pycnometer was calibrated with deionised double distilled water. The position of the liquid levels, in the two arms of the pycnometer (which should be air bubble-free), is recorded with the help of a travelling microscope. The uncertainty in the measurement of density is found to be 2 parts in 104 parts. The reproducibility in mole fractions was within ± 0.0002. The temperature was maintained by circulating water from a U10 thermostat controlled to ± 0.01 K.
The viscosity deviations (Δη) were calculated using
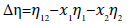 (2)
(2)
where η12 is the viscosities of the binary mixture.x1,x2 and η1,η2 are the mole fraction and the viscosities of pure components 1 and 2, respectively.
On the basis of the theories of absolute reaction rates [23], the excess Gibbs free energy of activation of viscous flow was calculated by using
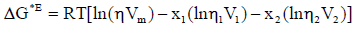 (3)
(3)
where η and Vm are the dynamic viscosity and molar volume of the mixture., η1,η2 and V1, V2 are viscosity and molar volume of pure components 1 and 2. R is the real gas constant and T is the absolute temperature.
The composition dependence of Δη and ΔG*E(YEcal) for each mixture are correlated by Redlich-Kister polynomial equation [24]:
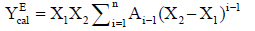 (4)
(4)
The coefficients of Ai-1 in the above equation along with the standard deviation σ (YE) have been calculated. These coefficients are the adjustable parameters to get best - fit values of YEcal .The standard deviations σ of YEcal were calculated by using the relation:
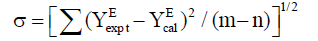 (5)
(5)
where m is the number of experimental data points and n is the number of coefficients considered and YEexpt,YEcal are the values of experimental and calculated property (Δη and ΔG*E) respectively.
There are several semi-empirical relations used to correlate the viscosity of binary liquid mixtures, which help us to know the strength of molecular interactions. The dynamic viscosities have been calculated by the following empirical relations.
The Grunberg–Nissan proposed the empirical relation as
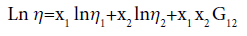 (6)
(6)
G12 may be regarded as an interaction parameter proportional to the interchange energy and is a measure of the strength of interaction between the component liquids 1 and 2.
Katti and Choudhri derived the following equation
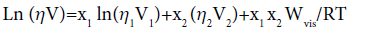 (7)
(7)
Here Wvis is an interaction term.
Heric and Brewer equation is
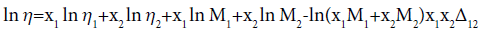 (8)
(8)
Where Δ12 is the interaction term and M1 and M2 are molecular weights of components 1 and 2.
Hind et al. suggested following equation for determination of the viscosity of the liquid mixtures as
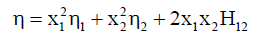 (9)
(9)
Where x1 and x2 are the mole fractions, η1 and η2 are the viscosities of liquid components 1 and 2 respectively. η is viscosity of binary mixture and H12 is Hind interaction parameter.
Dimethyl carbonate is a polar aprotic solvent having a dipole moment of 0.90D. Because of the electro negativity difference of carbon and oxygen atom of C=O group of DMC, it is expected to have dipoledipole interaction in its pure form. Alkoxyethanols are important class of glycolic ethers as they contain ether, alcohol and hydrocarbon chain in same molecule. This gives rise to formation of inter and intra molecular hydrogen bonds which makes them self-associated [25,26]. Alkanols represent an important class of hydrogen-bonded solvents, for which the degree of association is very sensitive to temperature and the presence of solvents [27]. Figure 1 shows the molecular structures of the chemical compounds used in this study.
The experimental values of viscosity, deviation in viscosity and excess Gibbs energy of activation of viscous flow for three binary mixtures DMC+MOE, DMC+EOE and DMC+BOE at different temperatures (303.15, 308.15, 313.15 and 318.15) K are presented in Table 2. Excess/deviation quantities are correlated by using Redlichkister polynomial Eq. (4) as a function of temperature. The fitting coefficients Ai-1 for all the three binary mixtures are listed in Table 3 along with their standard deviation σ (root mean square deviation) by using Eq. (5). From the excess/deviation properties, molecular interaction among the DMC (1)+MOE (2)+EOE (2)+BOE (2) has been interpreted.
| DMC+MOE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 303.15°K | 308.15°K | 313.15°K | 318.15°K | |||||||||
| x1 | η | Δη | ΔG*E | η | Δη | ΔG*E | η | Δη | ΔG*E | η | Δη | ΔG*E |
| (m Pa s) | (m Pa s) | (Jmol-) | (m Pa s) | (m Pa s) | (Jmol-) | (m Pa s) | (m Pa s) | (Jmol-) | (m Pa s) | (m Pa s) | (Jmol-) | |
| 0.0000 | 1.422 | 0.000 | 0.0000 | 1.248 | 0.000 | 0.0000 | 1.174 | 0.000 | 0.0000 | 1.079 | 0.000 | 0.0000 |
| 0.0940 | 1.326 | -0.014 | 51.3000 | 1.147 | -0.032 | -3.0800 | 1.063 | -0.046 | -41.5400 | 0.971 | -0.051 | -72.8500 |
| 0.1893 | 1.219 | -0.038 | 69.4000 | 1.052 | -0.058 | -8.1700 | 0.968 | -0.076 | -65.1100 | 0.879 | -0.085 | -126.9500 |
| 0.2859 | 1.109 | -0.063 | 64.2200 | 0.955 | -0.084 | -37.1100 | 0.876 | -0.101 | -102.1300 | 0.793 | -0.113 | -187.4800 |
| 0.3837 | 1.002 | -0.085 | 44.4000 | 0.862 | -0.106 | -77.9300 | 0.789 | -0.121 | -148.4900 | 0.715 | -0.131 | -246.8900 |
| 0.4830 | 0.901 | -0.099 | 14.8800 | 0.779 | -0.116 | -113.5900 | 0.711 | -0.131 | -191.3400 | 0.645 | -0.141 | -302.6000 |
| 0.5835 | 0.811 | -0.102 | -9.6100 | 0.702 | -0.120 | -154.1600 | 0.642 | -0.131 | -226.7600 | 0.585 | -0.140 | -341.8700 |
| 0.6855 | 0.736 | -0.088 | -10.7600 | 0.640 | -0.108 | -162.3400 | 0.585 | -0.117 | -235.6700 | 0.538 | -0.126 | -342.0800 |
| 0.7889 | 0.672 | -0.061 | 6.4300 | 0.593 | -0.079 | -126.2400 | 0.540 | -0.091 | -207.9600 | 0.501 | -0.100 | -306.3100 |
| 0.8937 | 0.612 | -0.030 | 20.5400 | 0.549 | -0.047 | -89.1100 | 0.506 | -0.053 | -137.9800 | 0.482 | -0.055 | -181.2400 |
| 1.0000 | 0.549 | 0.000 | 0.0000 | 0.518 | 0.000 | 0.0000 | 0.486 | 0.000 | 0.0000 | 0.473 | 0.000 | 0.0000 |
| DMC+EOE | ||||||||||||
| 0.0000 | 1.655 | 0.000 | 0.0000 | 1.483 | 0.000 | 0.0000 | 1.306 | 0.000 | 0.0000 | 1.231 | 0.000 | 0.0000 |
| 0.1131 | 1.465 | -0.065 | 12.2600 | 1.296 | -0.078 | -36.0000 | 1.098 | -0.115 | -156.4900 | 1.016 | -0.129 | -218.0200 |
| 0.2229 | 1.285 | -0.123 | -8.4200 | 1.124 | -0.144 | -100.6300 | 0.930 | -0.193 | -302.2500 | 0.840 | -0.222 | -439.7000 |
| 0.3297 | 1.120 | -0.170 | -55.2100 | 0.973 | -0.192 | -179.8300 | 0.795 | -0.241 | -432.9900 | 0.705 | -0.276 | -630.3800 |
| 0.4335 | 0.975 | -0.201 | -114.4400 | 0.841 | -0.224 | -272.3200 | 0.679 | -0.272 | -574.9500 | 0.600 | -0.302 | -792.6700 |
| 0.5344 | 0.859 | -0.205 | -152.9000 | 0.736 | -0.231 | -341.9200 | 0.598 | -0.270 | -646.0200 | 0.531 | -0.295 | -860.6300 |
| 0.6325 | 0.769 | -0.186 | -159.8000 | 0.660 | -0.213 | -357.6000 | 0.540 | -0.247 | -660.3200 | 0.482 | -0.270 | -869.6800 |
| 0.7281 | 0.705 | -0.145 | -115.0600 | 0.609 | -0.171 | -308.1100 | 0.500 | -0.209 | -617.0000 | 0.452 | -0.227 | -800.1100 |
| 0.8211 | 0.653 | -0.094 | -52.3600 | 0.567 | -0.124 | -243.4100 | 0.480 | -0.153 | -486.3700 | 0.436 | -0.173 | -662.6100 |
| 0.9117 | 0.602 | -0.045 | -9.0400 | 0.542 | -0.061 | -118.0900 | 0.470 | -0.088 | -311.2500 | 0.435 | -0.105 | -442.4800 |
| 1.0000 | 0.549 | 0.000 | 0.0000 | 0.518 | 0.000 | 0.0000 | 0.486 | 0.000 | 0.0000 | 0.473 | 0.000 | 0.0000 |
| DMC+BOE | ||||||||||||
| 303.15°K | 308.15°K | 313.15°K | 318.15°K | |||||||||
| x1 | η | Δη | ΔG*E | η | Δη | ΔG*E | η | Δη | ΔG*E | η | Δη | ΔG*E |
| (m Pa s) | (m Pa s) | (Jmol-) | (m Pa s) | (m Pa s) | (Jmol-) | (m Pa s) | (m Pa s) | (Jmol-) | (m Pa s) | (m Pa s) | (Jmol-) | |
| 0.0000 | 2.477 | 0.000 | 0.0000 | 2.172 | 0.000 | 0.0000 | 1.950 | 0.000 | 0.0000 | 1.709 | 0.000 | 0.0000 |
| 0.1472 | 1.915 | -0.278 | -57.2300 | 1.605 | -0.323 | -202.4400 | 1.400 | -0.334 | -298.5500 | 1.181 | -0.346 | -445.9300 |
| 0.2798 | 1.500 | -0.438 | -147.2700 | 1.235 | -0.474 | -365.0000 | 1.044 | -0.496 | -561.1700 | 0.852 | -0.511 | -837.7300 |
| 0.3997 | 1.209 | -0.497 | -222.2100 | 0.985 | -0.526 | -490.7300 | 0.821 | -0.544 | -739.6000 | 0.660 | -0.555 | -1092.2100 |
| 0.5088 | 1.001 | -0.495 | -278.4500 | 0.815 | -0.515 | -570.0900 | 0.672 | -0.533 | -860.8500 | 0.536 | -0.544 | -1266.2800 |
| 0.6084 | 0.865 | -0.439 | -269.2600 | 0.701 | -0.465 | -591.6200 | 0.584 | -0.475 | -867.2900 | 0.462 | -0.495 | -1322.3000 |
| 0.6998 | 0.760 | -0.368 | -255.2100 | 0.620 | -0.395 | -577.7400 | 0.521 | -0.405 | -841.5600 | 0.421 | -0.423 | -1265.1100 |
| 0.7838 | 0.690 | -0.276 | -190.5500 | 0.568 | -0.308 | -504.8300 | 0.482 | -0.321 | -751.8700 | 0.405 | -0.335 | -1093.7700 |
| 0.8614 | 0.628 | -0.188 | -148.0800 | 0.540 | -0.207 | -364.3900 | 0.465 | -0.224 | -579.6200 | 0.400 | -0.244 | -877.9000 |
| 0.9333 | 0.580 | -0.098 | -93.8400 | 0.527 | -0.101 | -180.9700 | 0.469 | -0.115 | -314.8200 | 0.422 | -0.133 | -509.9600 |
| 1.0000 | 0.549 | 0.000 | 0.0000 | 0.518 | 0.000 | 0.0000 | 0.486 | 0.000 | 0.0000 | 0.473 | 0.000 | 0.0000 |
Table 2: Viscosity, η (m Pa s), deviation in viscosity, Δη (m Pa s) and excess Gibbs energy of activation of viscous flow (ΔG*E) of the binary mixtures dimethyl carbonate and 2-alkoxyethanols at different temperatures.
| Property | Temp (K) | A0 | A1 | A2 | A3 | A4 | σ |
|---|---|---|---|---|---|---|---|
| DMC+MOE | |||||||
| Δη (mPa s) | 303.15 | -0.4008 | 0.1178 | 0.2456 | -0.0460 | 0.0079 | 0.0010 |
| 308.15 | -0.4687 | 0.1204 | 0.0662 | -0.0734 | -0.0209 | 0.0025 | |
| 313.15 | -0.5272 | 0.0738 | 0.0751 | -0.0925 | -0.1672 | 0.0005 | |
| 318.15 | -0.5666 | 0.0572 | -0.0104 | -0.1067 | -0.0336 | 0.0012 | |
| ΔG*E (Jmol-) | 303.15 | 190.8321 | 504.5860 | -562.2633 | -466.3476 | 1232.6103 | 2.9038 |
| 308.15 | -273.0796 | 752.7729 | -850.6797 | 129.0906 | 385.6151 | 8.6265 | |
| 313.15 | -572.6833 | 882.0853 | -773.5867 | 212.3205 | -282.5490 | 1.8102 | |
| 318.15 | -966.6345 | 1139.3057 | -1142.4131 | 305.1090 | 177.8822 | 5.8381 | |
| DMC+EOE | |||||||
| Δη (mPa s) | 303.15 | -0.8250 | 0.0185 | 0.4867 | -0.1008 | -0.2290 | 0.0009 |
| 308.15 | -0.9272 | 0.0473 | 0.4274 | -0.0882 | -0.2847 | 0.0027 | |
| 313.15 | -1.0883 | -0.0376 | 0.1275 | -0.0110 | -0.2798 | 0.0023 | |
| 318.15 | -1.1981 | -0.1816 | 0.0316 | 0.2782 | -0.2658 | 0.0016 | |
| ΔG*E (Jmol-) | 303.15 | -443.2362 | 1045.4785 | 319.6798 | -1223.4630 | 297.9004 | 4.5807 |
| 308.15 | -1088.1721 | 1695.1694 | 212.2916 | -1131.1650 | -415.5735 | 10.9696 | |
| 313.15 | -2231.3309 | 2178.2926 | -139.8629 | -223.5699 | -1629.7342 | 13.1814 | |
| 318.15 | -3125.3797 | 2334.5268 | 740.2032 | 748.9462 | -3590.6914 | 15.5163 | |
| DMC+BOE | |||||||
| Δη (mPa s) | 303.15 | -1.9748 | -0.5519 | 0.3246 | 0.2028 | -0.3346 | 0.0035 |
| 308.15 | -2.0594 | -0.5819 | -0.4166 | -0.0058 | 0.4304 | 0.0026 | |
| 313.15 | -2.1155 | -0.6726 | -0.4796 | 0.2323 | 0.3503 | 0.0031 | |
| 318.15 | -2.1938 | -0.5700 | -0.1163 | 0.0800 | -0.5251 | 0.0049 | |
| ΔG*E (Jmol-) | 303.15 | -1078.3654 | 505.3651 | 1320.6878 | 63.0246 | -1669.0766 | 10.7945 |
| 308.15 | -2206.4258 | 1033.6456 | -1217.4091 | -27.3645 | 1938.3759 | 10.4364 | |
| 313.15 | -3295.3255 | 1281.7176 | -1322.5151 | 816.1162 | 1552.7027 | 14.6838 | |
| 318.15 | -5067.7307 | 2394.6550 | 962.1371 | 194.9064 | -2909.7599 | 22.7845 | |
Table 3: Redlich-Kister coefficients Ai-1 and corresponding standard deviations (σ) computed for excess/deviation properties of the binary mixtures of DMC+alkoxyethanols (MOE, EOE, BOE) at different temperatures.
Viscosity deviation
The variation of deviation in viscosity, Δη, with mole fraction of DMC (x1) for the binary mixtures of MOE, EOE, BOE with DMC at T=(303.15, 308.15, 313.15 and 318.15) K is shown in Figure 2. The value and magnitude of Δη depend on molecular shape of the components in addition to intermolecular forces [28]. It is observed from the Figure 2a-2c that deviation in viscosity is negative for all the binaries and at all the temperatures. The absolute value of viscosity deviation of DMC+MOE, DMC+EOE and DMC+BOE systems increases linearly and reaches to a maximum value at x1~ 0.5835 for (DMC+MOE), x1~ 0.5344 for (DMC+EOE), x1~ 0.3997 for (DMC+BOE), after which gradually decreases until to a pure state of DMC. Generally, negative values of Δη indicate the presence of dispersion forces or mutual loss of specific interactions in molecules operating in the systems arising due to weak intermolecular interactions, and positive values of deviation in viscosity indicate strong specific interactions [29-31]. The dependence of Δη on composition for the binary mixtures under study may be explained in terms of physical, chemical and structural contributions [32,33].
i. Physical contributions: comprise dispersion forces and non-specific physical interactions and the sign of Δη may be negative;
ii. Chemical contributions consider the breaking up of hydrogen bond structure which gives negative Δη and specific interactions such as H-bond formation, charge-transfer complex formation and dipole– dipole interactions gives positive Δη values.
iii. Structural contribution: Difference in molar volumes and free volumes of liquid components, geometry of molecules, which favours fitting of the component molecules within the voids of each other, gives positive contribution.
According to Kaufman and Eyring [34], the viscosity of a mixture strongly depends on the entropy of mixture, which is related with the structure of the liquid. Vogel and Weiss [35] explained that mixtures with strong interactions between different molecules and negative deviations from Raoult’s law present positive viscosity deviations; whereas, for mixtures with positive deviations of Raoult’s law and without specific interactions the viscosity deviations are negative. The sign and magnitude of Δη depend on the combined effect of various factors such as molecular size, shape, and intermolecular forces. Emilio et al. presented negative values of viscosity deviation in the binary systems of dimethyl carbonate + 1-alcohols [36]. Mialkowski et al. reported negative ηE in the dimethyl carbonate and γ-butyrolactone (BL) [37], and they explained that the addition of DMC to BL involves a more important effect of breaking the structure. This means that interaction between pairs of like molecules is stronger than between pairs of unlike molecules. Anjali et al. discussed about intermolecular interactions between formamide and 2-alkoxyethanols in terms of viscometric study [19]. They inferred that 2-alkoxyethanol and formamide interaction predominate than those between 2-alkoxyethanol molecules which contributes to positive Δη values.
In the present study one can assume the formation hydrogen bonding among the binary liquids between the carbonyl group (C=O) of DMC and hydroxyl group (O-H) of alkoxyethanols. There is also possibility of dipole-dipole interactions among DMC and alkoxyethanols. If we consider structural contributions, the molar volumes of DMC, MOE, EOE and BOE are (85.246, 79.624, 97.830 and 132.452) g/cm3 respectively at 303.15 K. Hence interstitial accommodation of DMC in to the voids of MOE and EOE is difficult as they have similar molar volumes which makes expansion factor dominates and contributes to negative Δη values. Even though there is a possibility of interstitial accommodation of DMC into the voids of BOE, they give negative Δη values. This is explained well by the fact that alkoxyethanol molecules are being expected to exist in rings of 5/6 members [28] hence viscosity deviation remains negative. Because of the inter and intra molecular hydrogen bonds in alkoxyethanols, they are arranged in well-ordered manner and addition of DMC to the alkoxyethanols (MOE, EOE, BOE) may lead to disruption of liquid order on mixing and unfavourable interactions between unlike molecules producing negative contribution to Δη .The less negative Δη values for DMC+MOE system indicate that interactions are predominant over DMC+EOE and DMC+BOE systems. Thus, order of interaction follows MOE>EOE>BOE.
Excess Gibbs energy of activation of viscous flow
The excess Gibbs free energy of activation of viscous flow, like viscosity deviation, can be used to detect molecular interactions [38]. The variation of excess Gibbs energy of activation of viscous flow with mole fraction of DMC for the binaries MOE, EOE, BOE with DMC is shown in Figure 3. The excess Gibbs energy of activation of viscous flow is negative for DMC+EOE and DMC+BOE systems over the entire composition range and at all the temperatures. In case of DMC+MOE, at 303.15K ΔG*E values are positive at lower mole fractions of DMC. As the temperatures increases from 308.15K to 318.15K ΔG*E values becomes negative which affirm the weak specific interactions. It is well known fact that negative values of ΔG*E indicate the presence of weak physical forces in the system [39]. On the other hand, positive values of it suggest strong specific interactions (like hydrogen bonding and dipole-dipole interactions) between unlike molecules. Negative values of ΔG*E in the binary liquids of DMC with alkoxyethanols also support the conclusions drawn from viscosity deviation.
Analysing viscosity of liquid mixtures by semi empirical models
To interpret the molecular interaction in the liquid mixture, several empirical and semi empirical models (equations) have been put forth for correlating the viscosity of liquid mixtures. In this article, we use the equations of Grunberg–Nissan, Katti–Chaudhri, and Heric- Brewer and Hind et al. to correlate the viscosities of binary mixtures of DMC+alkoxyethanols (MOE, EOE, BOE).
Experimental and calculated values of viscosity (η)for the binary mixtures of dimethyl carbonate and alkoxyethanols (MOE, EOE, BOE) at the temperatures (303.15, 308.15, 313.15 and 318.15) K are presented in Tables 4a-4c. Interaction (adjustable) Parameters calculated from Eqs. (6-9) and the corresponding standard deviations (σ) for the binary mixtures of dimethyl carbonate and alkoxyethanols (MOE, EOE, BOE) at the temperatures (303.15, 308.15, 313.15 and 318.15) K are shown in Table 5.
| DMC+MOE | ||||||||||
| X1 | ηExpt | η GN | η KC | η HB | η H | ηExpt | η GN | η KC | η HB | η H |
| 303.15K | 308.15K | |||||||||
| 0.0000 | 1.422 | 1.422 | 1.422 | 1.422 | 1.422 | 1.248 | 1.248 | 1.248 | 1.248 | 1.248 |
| 0.0940 | 1.326 | 1.312 | 1.312 | 1.312 | 1.312 | 1.147 | 1.171 | 1.170 | 1.168 | 1.141 |
| 0.1893 | 1.219 | 1.208 | 1.207 | 1.207 | 1.206 | 1.052 | 1.093 | 1.091 | 1.089 | 1.041 |
| 0.2859 | 1.109 | 1.108 | 1.107 | 1.107 | 1.105 | 0.955 | 1.016 | 1.013 | 1.010 | 0.948 |
| 0.3837 | 1.002 | 1.013 | 1.012 | 1.012 | 1.009 | 0.862 | 0.939 | 0.935 | 0.933 | 0.862 |
| 0.4830 | 0.901 | 0.923 | 0.922 | 0.922 | 0.918 | 0.779 | 0.863 | 0.860 | 0.857 | 0.783 |
| 0.5835 | 0.811 | 0.838 | 0.837 | 0.837 | 0.833 | 0.702 | 0.789 | 0.786 | 0.784 | 0.713 |
| 0.6855 | 0.736 | 0.758 | 0.758 | 0.758 | 0.752 | 0.640 | 0.717 | 0.714 | 0.713 | 0.651 |
| 0.7889 | 0.672 | 0.683 | 0.683 | 0.683 | 0.678 | 0.593 | 0.647 | 0.646 | 0.645 | 0.597 |
| 0.8937 | 0.612 | 0.614 | 0.614 | 0.614 | 0.610 | 0.549 | 0.581 | 0.580 | 0.580 | 0.553 |
| 1.0000 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.518 | 0.518 | 0.518 | 0.518 | 0.518 |
| 313.15K | 318.15K | |||||||||
| 0.0000 | 1.174 | 1.174 | 1.174 | 1.174 | 1.174 | 1.079 | 1.079 | 1.079 | 1.079 | 1.079 |
| 0.0940 | 1.063 | 1.114 | 1.113 | 1.111 | 1.064 | 0.971 | 1.043 | 1.043 | 1.041 | 0.973 |
| 0.1893 | 0.968 | 1.049 | 1.047 | 1.044 | 0.963 | 0.879 | 0.999 | 0.997 | 0.995 | 0.876 |
| 0.2859 | 0.876 | 0.981 | 0.978 | 0.975 | 0.869 | 0.793 | 0.947 | 0.945 | 0.941 | 0.788 |
| 0.3837 | 0.789 | 0.910 | 0.907 | 0.904 | 0.785 | 0.715 | 0.888 | 0.886 | 0.882 | 0.711 |
| 0.4830 | 0.711 | 0.838 | 0.835 | 0.832 | 0.710 | 0.645 | 0.824 | 0.821 | 0.818 | 0.643 |
| 0.5835 | 0.642 | 0.765 | 0.762 | 0.760 | 0.644 | 0.585 | 0.756 | 0.754 | 0.751 | 0.586 |
| 0.6855 | 0.585 | 0.692 | 0.690 | 0.688 | 0.588 | 0.538 | 0.685 | 0.683 | 0.681 | 0.540 |
| 0.7889 | 0.540 | 0.621 | 0.620 | 0.618 | 0.543 | 0.501 | 0.613 | 0.612 | 0.611 | 0.505 |
| 0.8937 | 0.506 | 0.552 | 0.551 | 0.551 | 0.509 | 0.482 | 0.542 | 0.542 | 0.541 | 0.483 |
| 1.0000 | 0.486 | 0.486 | 0.486 | 0.486 | 0.486 | 0.473 | 0.473 | 0.473 | 0.473 | 0.473 |
Table 4a: Experimental and calculated values of viscosity, η (m Pa s), for the binary mixtures of dimethyl carbonate (DMC) and 2-methoxyethanol (MOE) at temperatures 303.15, 308.15, 313.15, 318.15K.
| DMC+EOE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | ηExpt | η GN | η KC | η HB | η H | ηExpt | η GN | η KC | η HB | η H |
| 303.15K | 308.15K | |||||||||
| 0.0000 | 1.655 | 1.655 | 1.655 | 1.655 | 1.655 | 1.483 | 1.483 | 1.483 | 1.483 | 1.483 |
| 0.1131 | 1.465 | 1.488 | 1.483 | 1.488 | 1.458 | 1.296 | 1.385 | 1.380 | 1.385 | 1.288 |
| 0.2229 | 1.285 | 1.337 | 1.328 | 1.337 | 1.284 | 1.124 | 1.280 | 1.272 | 1.280 | 1.120 |
| 0.3297 | 1.120 | 1.199 | 1.188 | 1.199 | 1.131 | 0.973 | 1.172 | 1.162 | 1.172 | 0.976 |
| 0.4335 | 0.975 | 1.074 | 1.063 | 1.074 | 0.999 | 0.841 | 1.064 | 1.054 | 1.064 | 0.855 |
| 0.5344 | 0.859 | 0.961 | 0.952 | 0.961 | 0.885 | 0.736 | 0.958 | 0.949 | 0.958 | 0.755 |
| 0.6325 | 0.769 | 0.860 | 0.852 | 0.860 | 0.788 | 0.660 | 0.857 | 0.850 | 0.857 | 0.674 |
| 0.7281 | 0.705 | 0.769 | 0.763 | 0.769 | 0.707 | 0.609 | 0.762 | 0.756 | 0.762 | 0.611 |
| 0.8211 | 0.653 | 0.687 | 0.683 | 0.687 | 0.641 | 0.567 | 0.673 | 0.670 | 0.673 | 0.565 |
| 0.9117 | 0.602 | 0.614 | 0.612 | 0.614 | 0.589 | 0.542 | 0.592 | 0.590 | 0.592 | 0.534 |
| 1.0000 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.518 | 0.518 | 0.518 | 0.518 | 0.518 |
| 313.15K | 318.15K | |||||||||
| 0.0000 | 1.306 | 1.306 | 1.306 | 1.306 | 1.306 | 1.231 | 1.231 | 1.231 | 1.231 | 1.231 |
| 0.1131 | 1.098 | 1.298 | 1.293 | 1.298 | 1.104 | 1.016 | 1.273 | 1.269 | 1.273 | 1.023 |
| 0.2229 | 0.930 | 1.257 | 1.249 | 1.257 | 0.935 | 0.840 | 1.270 | 1.263 | 1.270 | 0.850 |
| 0.3297 | 0.795 | 1.189 | 1.180 | 1.189 | 0.795 | 0.705 | 1.226 | 1.218 | 1.226 | 0.711 |
| 0.4335 | 0.679 | 1.101 | 1.092 | 1.101 | 0.683 | 0.600 | 1.150 | 1.140 | 1.150 | 0.602 |
| 0.5344 | 0.598 | 1.000 | 0.991 | 1.000 | 0.597 | 0.531 | 1.049 | 1.040 | 1.049 | 0.522 |
| 0.6325 | 0.540 | 0.892 | 0.885 | 0.892 | 0.534 | 0.482 | 0.933 | 0.926 | 0.933 | 0.468 |
| 0.7281 | 0.500 | 0.783 | 0.778 | 0.783 | 0.494 | 0.452 | 0.811 | 0.806 | 0.811 | 0.437 |
| 0.8211 | 0.480 | 0.677 | 0.674 | 0.677 | 0.473 | 0.436 | 0.690 | 0.688 | 0.690 | 0.429 |
| 0.9117 | 0.470 | 0.577 | 0.576 | 0.577 | 0.471 | 0.435 | 0.577 | 0.575 | 0.577 | 0.442 |
| 1.0000 | 0.486 | 0.486 | 0.486 | 0.486 | 0.486 | 0.473 | 0.473 | 0.473 | 0.473 | 0.473 |
Table 4b: Experimental and calculated values of viscosity, η (m Pa s), for the binary mixtures of dimethyl carbonate (DMC) and 2-ethoxyethanol (MOE) at temperatures (303.15, 308.15, 313.15, 318.15) K.
| DMC+BOE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | ηExpt | η GN | η KC | η HB | η H | ηExpt | η GN | η KC | η HB | η H |
| 303.15K | 308.15K | |||||||||
| 0.0000 | 2.477 | 2.477 | 2.477 | 2.477 | 2.477 | 2.172 | 2.172 | 2.172 | 2.172 | 2.172 |
| 0.1472 | 1.915 | 2.126 | 2.068 | 2.107 | 1.962 | 1.605 | 2.015 | 1.962 | 1.997 | 1.682 |
| 0.2798 | 1.500 | 1.815 | 1.735 | 1.789 | 1.566 | 1.235 | 1.810 | 1.732 | 1.784 | 1.314 |
| 0.3997 | 1.209 | 1.548 | 1.465 | 1.521 | 1.264 | 0.985 | 1.589 | 1.506 | 1.561 | 1.040 |
| 0.5088 | 1.001 | 1.320 | 1.245 | 1.296 | 1.035 | 0.815 | 1.374 | 1.298 | 1.349 | 0.840 |
| 0.6084 | 0.865 | 1.129 | 1.067 | 1.109 | 0.865 | 0.701 | 1.176 | 1.113 | 1.155 | 0.698 |
| 0.6998 | 0.760 | 0.969 | 0.921 | 0.954 | 0.741 | 0.620 | 1.000 | 0.952 | 0.985 | 0.602 |
| 0.7838 | 0.690 | 0.835 | 0.801 | 0.824 | 0.654 | 0.568 | 0.849 | 0.815 | 0.838 | 0.543 |
| 0.8614 | 0.628 | 0.722 | 0.701 | 0.716 | 0.596 | 0.540 | 0.719 | 0.699 | 0.713 | 0.513 |
| 0.9333 | 0.580 | 0.628 | 0.618 | 0.625 | 0.563 | 0.527 | 0.610 | 0.601 | 0.607 | 0.506 |
| 1.0000 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.518 | 0.518 | 0.518 | 0.518 | 0.518 |
| 313.15K | 318.15K | |||||||||
| 0.0000 | 1.950 | 1.950 | 1.950 | 1.950 | 1.950 | 1.709 | 1.709 | 1.709 | 1.709 | 1.709 |
| 0.1472 | 1.400 | 1.939 | 1.890 | 1.922 | 1.482 | 1.181 | 1.891 | 1.844 | 1.874 | 1.285 |
| 0.2798 | 1.044 | 1.820 | 1.744 | 1.793 | 1.134 | 0.852 | 1.901 | 1.824 | 1.873 | 0.974 |
| 0.3997 | 0.821 | 1.637 | 1.554 | 1.609 | 0.881 | 0.660 | 1.781 | 1.693 | 1.750 | 0.752 |
| 0.5088 | 0.672 | 1.429 | 1.353 | 1.403 | 0.701 | 0.536 | 1.584 | 1.501 | 1.555 | 0.598 |
| 0.6084 | 0.584 | 1.222 | 1.158 | 1.200 | 0.579 | 0.462 | 1.357 | 1.288 | 1.333 | 0.497 |
| 0.6998 | 0.521 | 1.029 | 0.981 | 1.013 | 0.502 | 0.421 | 1.130 | 1.079 | 1.113 | 0.439 |
| 0.7838 | 0.482 | 0.859 | 0.826 | 0.848 | 0.461 | 0.405 | 0.924 | 0.890 | 0.912 | 0.413 |
| 0.8614 | 0.465 | 0.712 | 0.693 | 0.706 | 0.448 | 0.400 | 0.745 | 0.725 | 0.738 | 0.414 |
| 0.9333 | 0.469 | 0.589 | 0.580 | 0.586 | 0.458 | 0.422 | 0.595 | 0.587 | 0.592 | 0.435 |
| 1.0000 | 0.486 | 0.486 | 0.486 | 0.486 | 0.486 | 0.473 | 0.473 | 0.473 | 0.473 | 0.473 |
Table 4c: Experimental and calculated values of viscosity, η (m Pa s), for the binary mixtures of dimethyl carbonate (DMC) and 2-butoxyethanol (EOE) at temperatures (303.15, 308.15, 313.15, 318.15) K.
An examination of data in Table 4 shows that all the empirical relations gave a reasonable fit, but the viscosity values calculated using Hind et al. are in good agreement with the experimental values. Perusal of data in Table 5 shows that the values of interaction parameters (d) calculated from different viscosity theories are positive for the systems: DMC+alkoxyethanols (MOE, EOE, BOE) at the four different temperatures.
According to Fort and Moore [40] if the G12 is positive, then the system exhibits strong interaction; if it is negative they show weak interaction. G12 may be regarded as an approximate measure of the strength of the interaction between the components Nigam and Mahl [41] concluded from the study of binary mixtures dimethylsulphoxide with chloroethanes and chloroethenes, that
i. if Δη >0,G12>0 and magnitude of both are large then strong specific interaction would be present;
ii. if Δη<0,G12>0 then weak specific interaction would be present;
iii. if Δη<0,G12<0 magnitude of both are large then the dispersion force would be dominant.
Interaction parameter Wvis/RT shows almost the same trend as that of G12. Infact, one could say that the parameters G12 and Wvis/ RT exhibit almost similar behaviour, which is not unlikely in view of logarithmic nature of both equations. In the present binary systems DMC + alkoxyethanols (MOE, EOE, BOE) G12 values in the Table 5, are less positive and viscosity deviation is negative (Δ
In the present investigation, the density and viscosity of the binary mixtures of dimethyl carbonate with 2-alkoxyethanols (MOE, EOE, BOE) have been experimentally measured at T=(303.15, 308.15, 313.15, 318.15) K. From the values of viscosity and density, deviation in viscosity and excess Gibbs free energy of activation of viscous flow are determined. The experimental values of viscosity were correlated with the semi empirical relations of viscosity like Grunberg-Nissan, Katti- Chaudhri, Heric-Brewer, and Hind et al. Among all the relations Hind et al. relation gave good agreement with the experimental values. From the observed negative values of Δ𝜂, negative values of ΔG*E and low positive values of G12 interaction parameter, it is concluded that weak specific interactions are present among the studied binary liquids.
One of the authors, Sk. Beebi wishes to thank Department of Chemistry, Acharya Nagarjuna University for providing laboratory facilities.