Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Research Article - (2021)
A mosaic of various inflammatory diseases associated with the administration of vaccines has been reported in the literature. Optic Neuritis (ON), a primary inflammation of the optic nerve, serves as an prominent piece of it, presenting the features of autoimmune phenomena including Treg/Th17 cell imbalance and the abundance of proinflammatory cytokines. A PubMed search revealed 48 cases of vaccination-related ON, from which 32 were reports as isolated ON, 9 occurred as the first symptom of neuromyelitis optica spectrum diseases (NMOSD) and 6 were inscribed in clinical course of Acute Disseminated Encephalomyelitis (ADEM). In Vaccine Adverse Effects Reporting System (VAERS) 537 reports of ON were identified, the vast majority of them was isolated (n=284), followed by ON preceding multiple sclerosis (n=99), ADEM (n=30) and NMOSD (n=20). Influenza vaccine was the leading cause of isolated ON, hepatitis B vaccine (HBV) was mostly associated with diseases entailing further demyelination, such as multiple sclerosis. Moreover, the time interval between vaccine delivery and symptoms of ON occurrences was shorter in cases of isolated-ON in comparison to ON-MS and ON-NMOSD. This time gap was also considerably longer after vaccines enriched with aluminum adjuvant. This article presents the thorough analysis of vaccination related ON cases and focuses on the possible pathomechanism of this autoimmune interplay, including the impact of adjuvants and the mechanism of molecular mimicry.
Optic neuritis; Vaccines; Autoimmune syndrome induced by adjuvants; Multiple sclerosis; Neuromyelitis optica; Acute disseminated encephalomyelitis
Optic neuritis (ON) is an inflammatory demyelinating disease of the optic nerve. The typical clinical characteristics of ON include reduced visual acuity and peripheral vision, decreased perception of brightness accompanied by globe tenderness and pain during eye movement [1]. ON is infrequent disease, the incidence varies from 0.94 to 2.18 per 100 000 per year regarding the unilateral form of the disease, bilateral subtype of ON occurs even less often [2]. The pathogenesis of ON remains poorly characterized, although many facts combine in favor of autoimmune nature of this disorder. ON may develop in the course of several Central Nervous System (CNS) demyelinating autoimmune diseases, with MS as the leading cause (15%-20% of cases) [3], followed by Neuromyelitis Optica Spectrum Disorders (NMOSD), and Acute Disseminated Encephalomyelitis (ADEM). ON can be also inscribed in the course of systemic inflammatory connective tissue disorders, such as systemic lupus erythematosus (SLE), systemic sclerosis or Behcet's disease [4,5], although infrequently [4]. Furthermore, mechanisms underlying autoimmune reactions including Treg/Th17 cell imbalance [6], an abundance of proinflammatory cytokines [7], the presence of circulating autoantibodies combined with the underlying genetic background of autoimmunity (HLA- DQB1*06:02-HLA-DRB1*15:01 haplotype [8] were reported in ON patients. The etiology of most autoimmune processes is multi-factorial, combining genetic, immunological, hormonal and environmental factors forming the “mosaic of autoimmunity” [9]. Infectious antigens play a key role in this interplay, and ON, especially in the pediatric population, may be associated with a preceding infectious illness, both of viral and bacterial etiology [10], including influenza [11], mumps [12], varicella zoster [13], cytomegalovirus [14], herpesvirus-6 [15], Epstein–Barr virus [16] and mycoplasma pneumoniae [17]. In most cases, symptoms of ON begin after the patient had recovered from the infectious disease, suggesting an autoimmune reaction to the pathogen as a trigger of the inflammatory response. Several cases of ON following vaccination were also reported [10]. The aim of this study was to examine and determine the clinical characteristics of vaccination- related ON.
SA literature search was performed using MEDLINE [PubMed] database, employing as keywords: "optic neuritis"; "vaccine"; "vaccination"; "vaccine-associated". Article bibliographies were checked to ensure all studies and reports were included. Moreover, we conducted the Vaccine Adverse Event Reporting System (VAERS) database search for reports of ON onset following any type of vaccination, using "optic neuritis" and "optic neuritis retrobulbar" as the keywords. The analysis included the reports that were obtained in the period January 1990-January 2019. Based on the symptoms reported, we have classified ON cases into the following categories:
• Isolated ON
• ON preceding NMOSD
• ON preceding ADEM
• ON preceding MS
• ON in the course of other diseases
Case reports and case series
An extensive literature review found 48 cases of vaccine-related ON reported in the years 1949-2018. From this group, the majority (32 cases) presented with the clinical features of isolated ON, followed by ON in course of NMOSD in 9 patients and ADEM in 6. ON generally occurred after the booster dose of the vaccine, although cases of the autoimmune phenomenon after the first dose of the vaccine were also noted. It is important to notice that the quality and detail of reported cases varied, mostly due to the indisputably long time interval between first and latest reports and therefore diagnostic capabilities discrepancy. Moreover, the observation time of individuals affected by ON was relatively short and did not exceed two years. Consequently, the incidence of patients in whom ON was the first symptom of the central nervous system inflammatory demyelinating disease might have been underestimated.
Isolated optic neuritis
Isolated ON occurred in 32 patients, equally often in women than in men. The time interval between the vaccination and the occurrence of the first ON symptoms ranged between 6 hours to 21 days with a mean number of 12.05 days. ON was most frequently associated with the vaccination against influenza (7 cases), followed by measles, mumps, rubella vaccine (MMR; 4 cases+ 1 without the mumps antigen), dyptheria, tetanus, pertussis vaccine(DTP; 4 cases), Hepatitis B Virus vaccine (HBV; 3 cases) and vaccines against Hepatitis A Virus (HAV), Varicella Zoster Virus (VZV), anthrax, rabies (2 cases each). In 4 cases ON was a result of multiple vaccinations (DTP+IPV, DTP+ smallpox, HAV+ typhoid fever). The course of ON was bilateral in 69% of cases, unilateral in 31% of them. Interestingly, the vaccine against influenza was associated with bilateral ON exclusively. The outcome of ON treatment was successful in the majority of patients: 56% fully regained their vision, in 12.5% partial improvement was observed. In 12.5% of the affected individuals, there was no recovery.
Optic neuritis preceding NMOSD
NMOSD (previously known as Devic’s disease) form a group of autoimmune, demyelinating disorders of CNS with typical clinical manifestations of optic neuritis and acute transverse myelitis attacks. Previously believed to be a variant of MS, it is now considered an independent disorder. The demyelination process in the course of NMOSD is associated with the presence of autoantibodies against aquaporin-4 (AQP4-IgGs). Nevertheless, AQP4-IgG seronegativity in 10%-25% of NMO patients suggests that there are several other factors involved in NMO immunopathogenesis, i.e., autoantibodies against aquaporin-1 (AQP1-Abs) and antibodies against myelin oligodendrocyte glycoprotein (MOG-IgGs) [18,19]. Post-vaccination ON led to NMOSD diagnosis in 9 patients, 4 men and 5 women. The time interval between vaccination and first symptoms of ON was significantly longer than in isolated ON, ranging from 3 days to 9 months with the mean of 99 days. All cases in which ON occurred after the period of one month were associated with HPV vaccination. HPV vaccine was also the leading cause of ON in this group: symptoms of NMOSD developed in 4 patients, followed by vaccines against pneumococcal infections, yellow fever, HBV, DTP, Japanese encephalitis (1 case each). The course of disease was unilateral in 5 patients and bilateral in 4. The presence of AQP4-IgG antibodies was confirmed in 2 out of 9 individuals, anti-MOG antibodies were positive in 3, although their incidence may be underestimated, as in 3 cases the results of the test were not reported. Although signs of recovery were observed in all affected patients, the clinical cause of the disease was more aggressive and therefore required intensified treatment regimen, consisting of steroids, plasma exchanges and disease-modifying antirheumatic drugs (DMARDs).
ON preceding ADEM
ADEM is an immune-mediated demyelinating CNS disorder, characterized clinically by new-onset polyfocal neurologic symptoms including encephalopathy, coupled with neuroimaging evidence of multifocal demyelination. ADEM is classically considered a monophasic illness, with the highest incidence in early childhood. As early as in the 18th-century temporal relationship with ADEM onset and infections was observed. Over a century later, an association of ADEM with vaccines, notably rabies, was reported [20]. In the literature we identified 6 cases of ON inscribed in the course of ADEM. The mean time interval between vaccination and ON was 20 days. Female to male ratio in ON-ADEM population was 1:1. The clinical course of ON was bilateral in 5 patients, unilateral in 1. Two cases of ON were reported as side effects of vaccines against influenza; rubella, rabies, HPV and MMR vaccines were related to 1 case of ON each.
Cases of ON reported in VAERS
VAERS, co administered by the Centers for Disease Control and Prevention (CDC) and United States Food and Drug Administration (FDA) is a passive surveillance system collecting reports of adverse effects following vaccination from healthcare providers, vaccine manufacturers and affected individuals themselves [21]. VAERS data include demographic information about vaccine recipients, specific vaccine received, details of experienced adverse effect and patients’ medical histories [22]. It is important to emphasize that VAERS has many limitations. To begin with, VAERS collects data on any adverse effect following vaccination, including coincidental ones and truly caused by the vaccine. The quality and completeness of VAERS reports is variable and many lack variable medical diagnoses. Moreover, mild reactions following vaccination are underrepresented in the database, serious ones on the other hand may be overreported by patients in response to intense media attention and increased public awareness [21].
In the VAERS database, we have identified 537 reports of vaccination-related ON, from which 448 were qualified for further analysis and 89 were excluded due to lack of final medical diagnosis. From the 448 VAERS reports 362 referred to single post-vaccination reaction cases (had unique VAERS identity number).
Isolated ON was the most frequently reported (229 cases), followed by ON proceeding of MS (85 cases: in 79 ON was the first symptom of MS, in 6 vaccinations led to disease exacerbation presenting as ON). In 20 cases ON occurred as the symptom of ADEM, in 14 appeared in the clinical spectrum of NMOSD. In 7 cases ON preceded the development of rheumatic disease (Behcet’s disease, rheumatoid arthritis, giant cell arteritis, SLE, macrophagic myofascitis, undifferentiated connective tissue disease), in 2 it was a prelude to other autoimmune diseases (Hashimoto’s thyrioditis, ulcerative colitis). 4 cases were related to the development of neurological impairment (Guilllain – Barre syndrome, HPV vaccination-associated neuro-immunopathogenic syndrome – HANS [23]), in one case autoimmune syndrome induced by adjuvants (ASIA) [24] was diagnosed. The vaccination – ON onset interval was considerably longer after aluminum-adjuvanted vaccines (mean 52.11 days,) in comparison to vaccines not enriched with this chemical element (mean 22.28 days).
Isolated ON
Isolated ON was reported 284 times, which correspond to 229 single cases of post-vaccination ON. ON was more likely to be observed after vaccination with viral vaccine (230 reports) than bacterial one (54 reports). Individuals affected were mainly of female gender (female: male ratio 2:1), the course of ON was unilateral in 104 cases and bilateral in 55. Vaccines most frequently associated with ON were influenza (63 reports), HBV (56 reports) and HPV (47 reports) (Figure 1). The mean time interval between vaccination and ON development was 25.83 days (Figure 2). Underlying allergic or autoimmune conditions were reported in 18 and 3 individuals from the affected population, respectively.
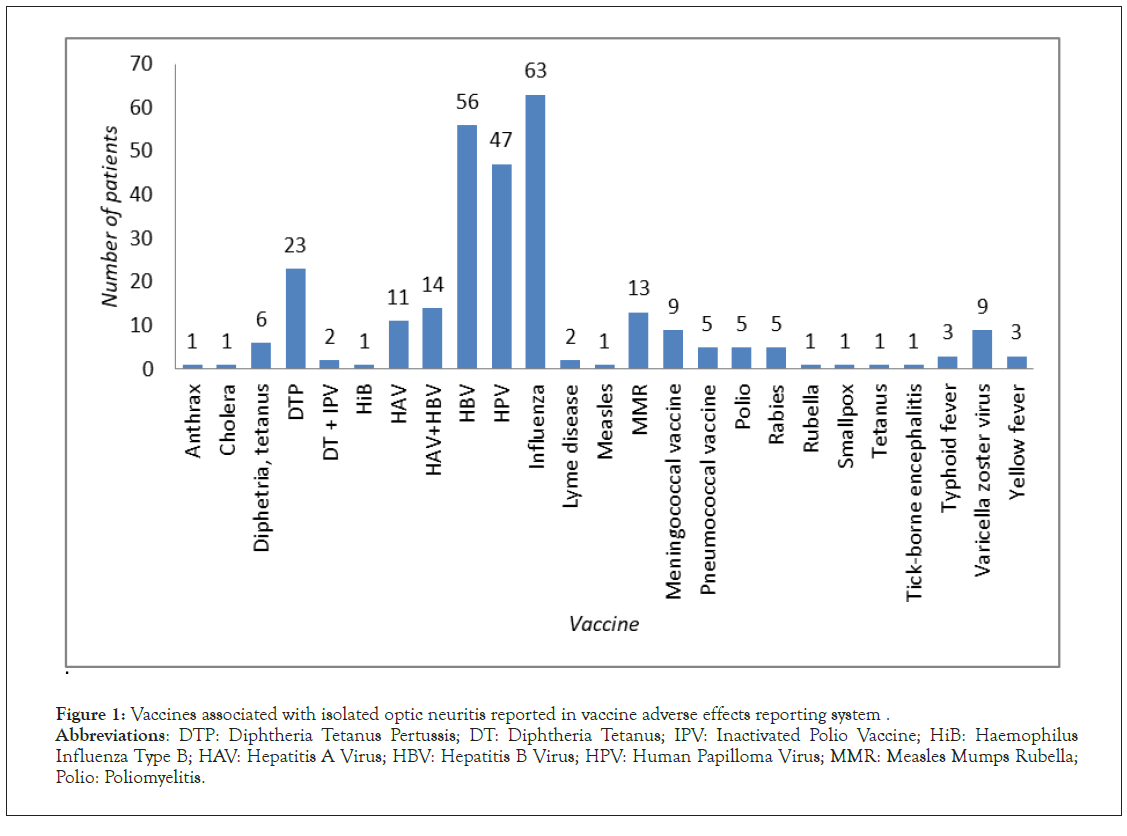
Figure 1: Vaccines associated with isolated optic neuritis reported in vaccine adverse effects reporting system.
Abbreviations: DTP: Diphtheria Tetanus Pertussis; DT: Diphtheria Tetanus; IPV: Inactivated Polio Vaccine; HiB: Haemophilus
Influenza Type B; HAV: Hepatitis A Virus; HBV: Hepatitis B Virus; HPV: Human Papilloma Virus; MMR: Measles Mumps Rubella;
Polio: Poliomyelitis.
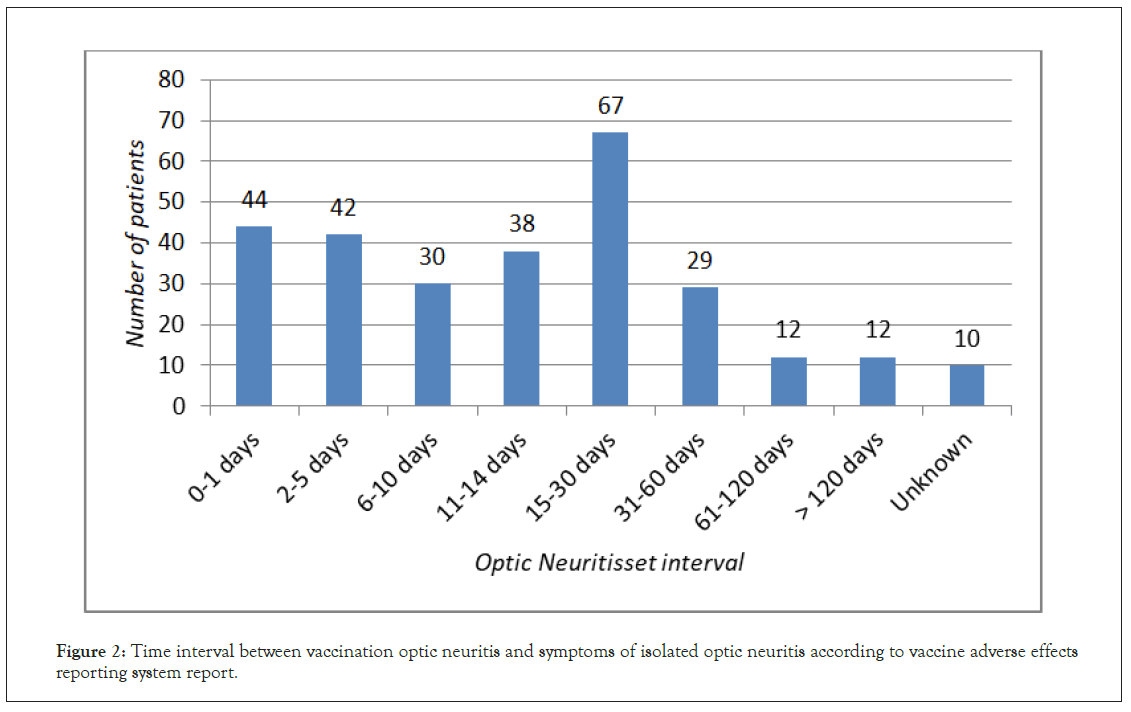
Figure 2: Time interval between vaccination optic neuritis and symptoms of isolated optic neuritis according to vaccine adverse effects reporting system report.
ON associated with MS
ON preceding MS was reported 93 times, what corresponded to 85 clinical cases: 79 in which ON was the first symptom of developing MS and 6 in which vaccination led to MS exacerbation, presenting as ON. The vast majority of VAERS reports was associated with vaccinations against viral pathogens (85 reports), with the HBV vaccination (54 reports) as the leading cause, followed by HPV (10 reports) and Influenza (5 reports) (Figure 3). The time interval between the vaccination and ON development was significantly longer than in other cases of ON with the mean of 78.57 days (Figure 4). ON appeared more frequently in females than in males (65 versus 18 cases) and was mainly unilateral.
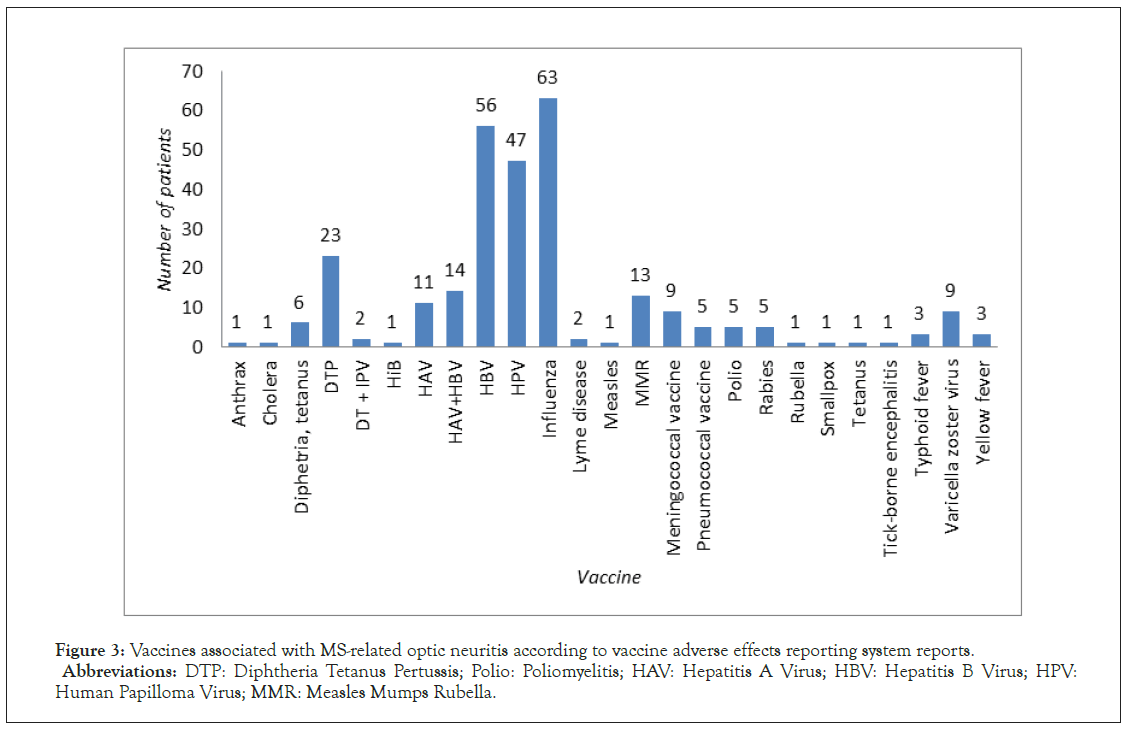
Figure 3: Vaccines associated with MS-related optic neuritis according to vaccine adverse effects reporting system reports.
Abbreviations: DTP: Diphtheria Tetanus Pertussis; Polio: Poliomyelitis; HAV: Hepatitis A Virus; HBV: Hepatitis B Virus; HPV:
Human Papilloma Virus; MMR: Measles Mumps Rubella.
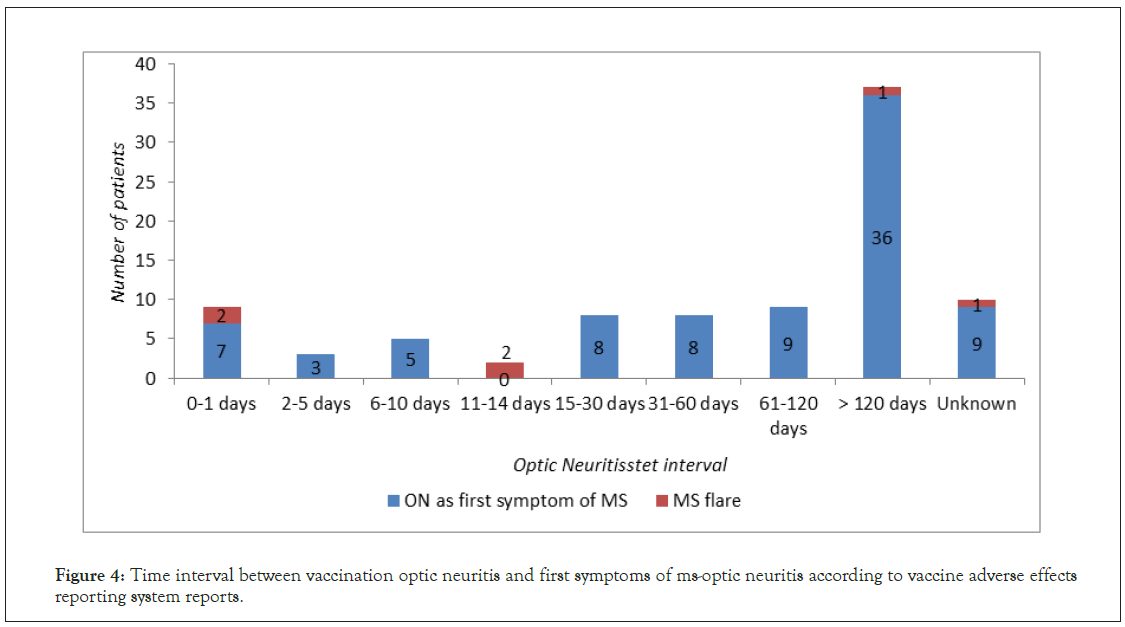
Figure 4: Time interval between vaccination optic neuritis and first symptoms of ms-optic neuritis according to vaccine adverse effects reporting system reports.
ON preceding ADEM
ON preceding ADEM was reported 30 times, what corresponds to 20 cases of this phenomenon. 16 out of 30 vaccination-related ON reports concerned multiple vaccinations. The vaccine most frequently associated with ON in this subgroup was DTP (6 reports), followed by HBV (5 reports) and Influenza (5 reports). The mean time interval between vaccination and development of ON was 18.66 days (Figure 5). There was no significant difference in ON cases sex distribution or course of the disease.
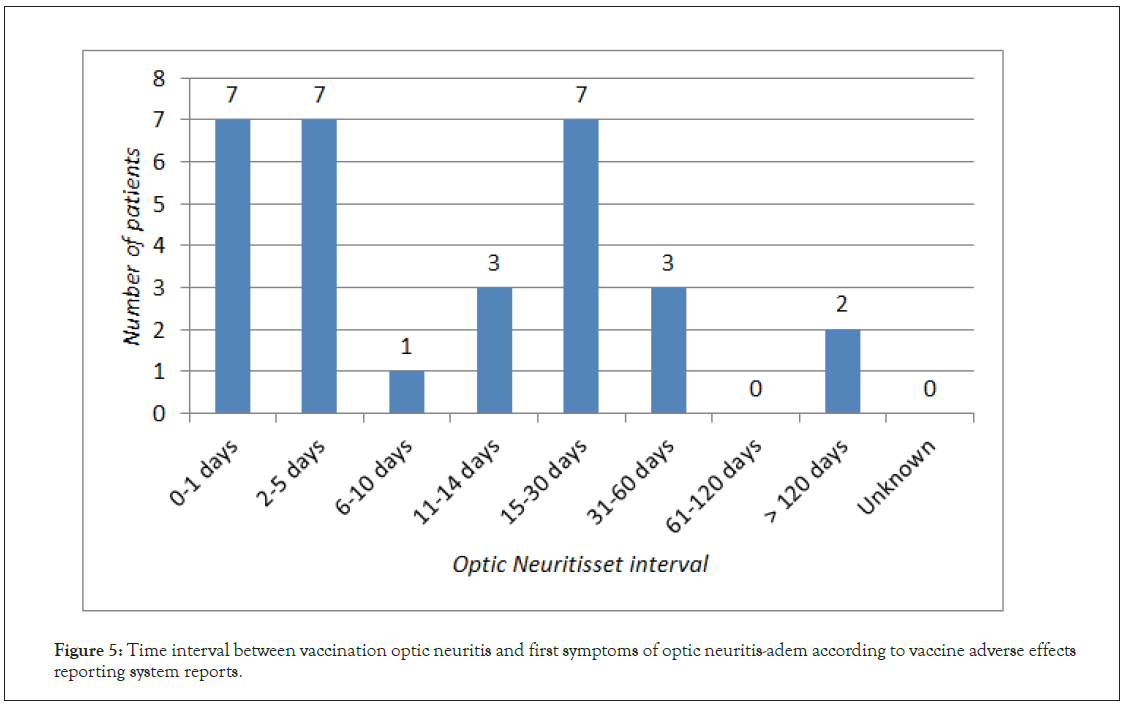
Figure 5: Time interval between vaccination optic neuritis and first symptoms of optic neuritis-adem according to vaccine adverse effects reporting system reports.
ON preceding NMOSD
ON appearing as part of NMOSD was reported 20 times, in 14 patients, 10 females and 4 males. The mean time interval between vaccination and ON was 41.93 days (Figure 6). Vaccination against viral pathogen preceded symptoms of NMOSD in 13 reports, in 7 the reported vaccine was against bacterial pathogen. The course of the disease was mainly bilateral (9 reports). There was no vaccine predominantly associated with NMOSD onset, ON was observed after HPV vaccination in 4 reports, after DTP in 3 and in two cases after HBV, influenza and meningococcal vaccines.
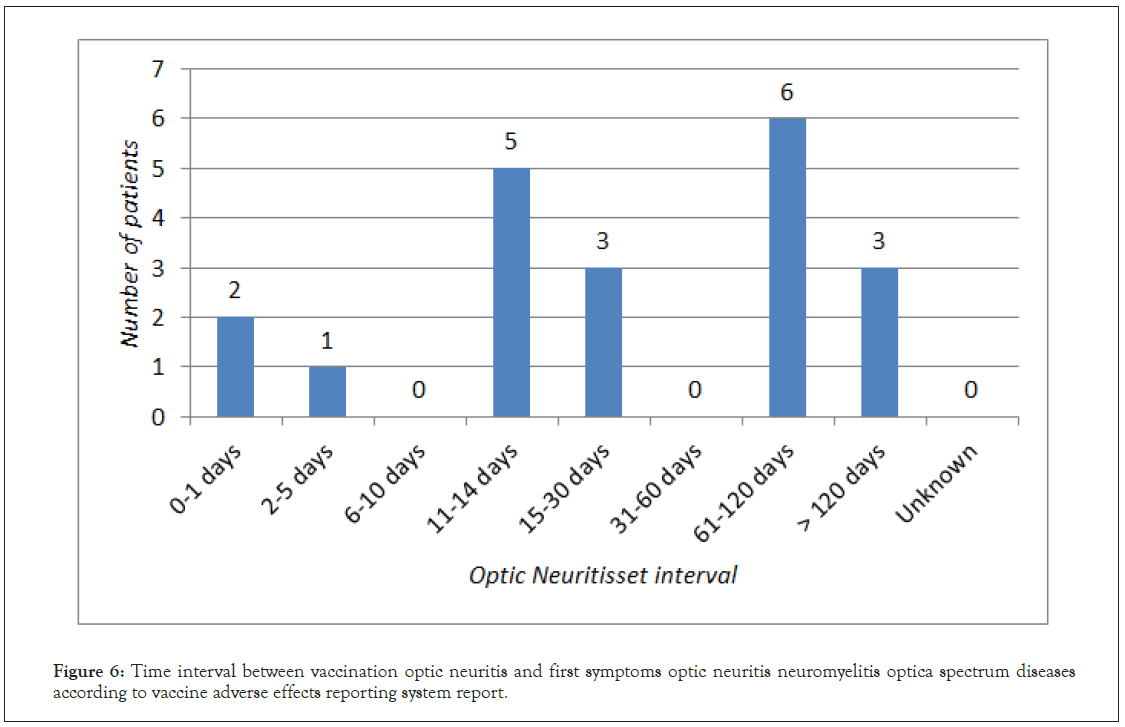
Figure 6: Time interval between vaccination optic neuritis and first symptoms optic neuritis neuromyelitis optica spectrum diseases according to vaccine adverse effects reporting system report.
ON following vaccination, although frequently reported in the literature (Table 1) remains the phenomenon with elusive underlying mechanism. In our study we have observed that vaccination-related ON is more likely to develop after vaccines against viral pathogens and that different types of vaccines are associated with distinct ON-associated diseases. Isolated ON was mostly reported following vaccination against influenza, whereas in ON-MS HBV vaccine was the leading cause. Isolated ON was the most frequent manifestation of demyelinisation both in cases reported in the literature and in VAERS, although its incidence might have been overestimated, as MS may be diagnosed in this population even years after the first episode of ON [25]. Moreover, reports of many ON cases lacked the full antibody screening (anti-AQP4, anti-MOG, anti-ganglioside, anti-phosphatidylcholine [26]. The time interval between vaccination and symptoms of ON was shortest in the isolated ON subgroup, followed by ADEM and was significantly prolonged in cases of NMOSD and MS-ON, suggesting different factors involved in the pathogenesis of those clinical entities. We have also observed, that vaccines containing aluminum were associated with delayed onset of ON, in comparison to vaccines devoid of this adjuvant. Although numerous reports suggest that demyelination may be associated with various types of vaccines the epidemiological studies on that topic failed to show the association [27,28].
| No. | Year | Vaccine | Brand name of vaccine | Uni/ Bilateral |
Day interval | Age/ Sex | Other symptoms | Other symptoms | Outcome/ follow up | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 1949 | Diphteria | N/R | Bilateral | N/R | N/R | Ophtalmoplegia | N/R | N/R | N/R | ON | [57] |
| 2. | 1953 | Smallpox, Diphteria, Tetanus | N/R | Bilateral | N/R | N/R | N/R | N/R | N/R | N/R | ON | [58] |
| 3. | 1975 | MMR | N/R | Bilateral | 20 days | 6/ M | General malaise | N/R | N/R | N/R | ON | [59] |
| 4. | 1979 | Influenza | N/R | Bilateral | N/R | N/R | N/R | Steroids | Recovery | N/R | ON | [60] |
| 5. | 1979 | Rubella | N/R | Bilateral | 5 days | 3/ F | Myelitis | N/R | N/R | N/R | ADEM | [61] |
| 6. | 1991 | BCG | N/R | Bilateral | 5 days | 12/F | N/R | Steroids | Recovery | N/R | [62] | |
| 7. | 1992 | Tetanus booster | N/R | Bilateral | 3 days | 11/F | Spastic paraparesis | Steroids, IVIG | Recovery | N/R | NMOSD | [63] |
| 8. | 1996 | Influenza | N/R | Bilateral | N/R | 61/F | N/R | Steroids | Recovery | N/R | ON | [64] |
| 9. | 1996 | MMR | N/R | Bilateral | 21 days | 13/M | Absent | Steroids | No recovery | N/R | ON | [65] |
| 10. | 1996 | MMR | N/R | Unilateral | 18 days | 13/ F | Absent | Steroids | Partial recovery | N/R | ON | [65] |
| 11. | 1997 | Influenza | N/R | Bilateral | 14 days | 59/F | Absent | N/R | N/R | N/R | ON | [66] |
| 12. | 1997 | HBV | N/R | Bilareral | N/R | N/R | N/R | N/R | N/R | N/R | ON | [67] |
| 13. | 1998 | Influenza | N/R | Bilateral | N/R | N/R | N/R | N/R | No recovery | N/R | ON | [68] |
| 14. | 1999 | Polio, HBV | N/R | Bilateral | 7 days | 44/F | Absent | Steroids | No recovery | N/R | ON | [69] |
| 15. | 2001 | Tdap- IPV | N/R | Unilateral | 10 days | 56/F | Absent | Steroids | Recovery | N/R | ON | [70] |
| 16. | 2001 | Rabies | Semple (9 doses) | Unilateral | 9 days | 31/M | Spastic paraparesis | Steroids | Recovery | N/R | ON | [71] |
| 17. | 2002 | Anthrax | N/R | Unilateral | 1 day | 39/ M | Absent | Steroids | Recovery | N/R | ON | [72] |
| 18. | 2002 | Anthrax | N/R | Bilateral | 14 days | 23/M | Absent | Steroids, azathioprine | Recovery | N/R | ON | [72] |
| 19. | 2004 | Rabies | N/R | Bilateral | 10 days | 15/M | Absent | Steroids | Recovery | N/R | ON | [71] |
| 20. | 2004 | Measles, Rubella | N/R | Bilateral | 6 hours | 16/ M | Absent | Steroids | Partial recovery | N/R | ON | [73] |
| 21. | 2004 | Rabies | N/R | Bilateral | 9 days (9 doses) | 45/M | Retention of urine, paraesthesia, hipotonia ( 1 month before optic neuritis) | Steroids | Recovery | N/R | ADEM | [74] |
| 22. | 2005 | BCG | N/R | Bilateral | N/R | N/R | N/R | N/R | N/R | N/R | ON | [75] |
| 23. | 2005 | Influenza | Fluvax | Bilateral | 21 days | 61/M | Hypersomnolance, delirium 2 months after optic neuritis, | Steroids iv, steroids po | No improvement regarding optic neuritis, neurological symptoms resolved | N/R | ADEM | [76] |
| 24. | 2006 | Men C | N/R | Bilateral | N/R | 13/ M | N/R | Iv steroids | Recovery In one eye | N/R | ON | [77] |
| 25. | 2008 | HPV | Gardasil® (3rd dose) | Unilateral | 9 months | 17/F | Right leg monoparesis (4 months after vaccination) | Steroids, plasma exchange, Rituximab | N/R | NMO IgG positive | NMOSD | [78] |
| 26. | 2008 | HPV | Gardasil® (3rd dose) | Unilateral | 5 months | 14/F | Right thigh dysesthesias 4 months after ON | Steroids, Rituximab, MMF | Recovery | NMO IgG positive | NMOSD | [78] |
| 27. | 2009 | H1N1 Influenza | Arepanrix® | Bilateral | 6 days | 2/M | Unstedy gait | Steroids | Recovery | N/R | ADEM | [79] |
| 28. | 2009 | HPV | Gardasil® | Unilateral | 8 months | 18/F | Leg weakness, back pain | N/R | N/R | N/R | NMOSD | [78] |
| 29. | 2009 | HAV, attenuated | N/R | Unilateral | 12 days | 39/M | Absent | Steroids | Recovery | HIV positive, lymphocytic pleocytosis In CSF | ON | [80] |
| 30. | 2009 | HBV | N/R | Unilateral | 7 days | 9/ F | Absent | Steroids | Recovery | N/R | ON | [81] |
| 31. | 2010 | HPV | N/R | Bilateral | 10 days | 16/F | Left hemiparesis | Steroids , plasma exchange | No improvement regarding optic neuritis, recovery from neurological symptoms | N/R | ADEM | [82] |
| 32. | 2010 | Japanese encephalitis | N/R | Bilateral | “few” days | 15/M | Urinary retention, tetraplegia with sensory disturbance |
Steroids | Recovery | HLA-DPB1*0501 allele positive | NMOSD | [83] |
| 33. | 2012 | Influenza (nasal) | N/R | Bilateral | N/R | 13/M | Absent | Steroids | Improvement after 2 months | N/R | ON | [84] |
| 34. | 2012 | Pneumococcal 23-valent polysachiride vaccination | N/R | Bilateral | “few” days | 87/ M | Paraplegia, hiccups | Steroids | Visual field improvement, no recovery from neurological symptoms | AQP4-IgG positive | NMOSD | [85] |
| 35. | 2012 | Trivalent Influenza Vaccine | Vaxigrip® | Bilateral | 14 days | 18/M | Absent | Steroids | Recovery | N/R | ON | [86] |
| 36. | 2013 | Typhoid fever, HAV | N/R | Bilateral | 14 days | 51/ M | Absent in the observation period of 2 years | Steroids, IVIG, plasma exchange | No recovery | AQP4-IgG negative | ON | [87] |
| 37. | 2014 | MMR | N/R | Unilateral | 10 weeks | 6/ F | right hemiparesis and right facial palsy |
Steroids | Recovery | AQP4-IgG negative | ADEM | [88] |
| 38. | 2014 | VZV | Zostavax | Bilateral | 21 days | 55/ F | Absent | Steroids | Recovery | AQP4-IgG negative | ON | [89] |
| 39. | 2014 | VZV | Zostavax | Unilateral | 7 days | 44/ F | Absent | Steroids | Recovery | AQP4- IgG negative | ON | [89] |
| 40. | 2015 | HBV, Tdap | N/R | Bilateral | 11 days | 28/M | Ascending numbness, ataxia | Steroids, plasma exchange, MMF | Recovery | AQP4-IgG negative | NMOSD | [90] |
| 41. | 2016 | Yellow fever | N/R | Unilateral | 14 days | 23/M | Allodynia, hyperalgesia of the right arm, erythrema | Steroids, Rituximab | Recovery | AQP4-IgG positive | NMOSD | [91] |
| 42. | 2016 | HPV (II dose) | Gardasil® | Unilateral | 7 days | 30/F | Absent | Steroids | Partial recovery | NMO IgG positive | NMOSD | [92] |
| 43. | 2016 | MMR | N/R | Unilateral | 7 days | 30/F | Absent | Steroids | Recovery | N/R | ON | [93] |
| 44. | 2017 | Tdap | Boostrix® | Unilateral | 21 days | 38/ F | Absent | Steroids | Recovery | AQP4-IgG and MOG IgG negative | ON | [94] |
| 45. | 2017 | Tdap | Boostrix® | Unilateral | 14 days | 38/ F | Absent | None | Spontaneous recovery | AQP4-IgG and MOG IgG negative | ON | [94] |
| 46. | 2018 | DTaP-IPV | N/R | Bilateral | 1 day | 27/M | Absent | Steroids | Recovery | CSF myelin basic protein positive, but negative for oligoclonal bands and neuromyelitis optica autoantibody serology |
ON | [95] |
| 47. | 2018 | Influenza, inactivated | N/R | Bilateral | 14 days | 23/F | Absent | Steroids | Recovery | AQP4-IgG negative, craniotomy from the right frontal lobe, which showed reactive astrogliosis with lymphocytic infiltration |
ON | [96] |
| 48. | 2018 | HPV (1st dose) | N/R | Bilateral | 21 days | 13/F | Absent | Steroids | Recovery | N/R | ON | [97] |
Abbreviations: ON: Optic Neuritis; ADEM: Acute Disseminated Encephalomyelitis; NMOSD: Neuromyelitis Optica Spectrum Disorders; N/R: Not Reported; F: Female; M: Male; MMR: Measles Mumps Rubella Vaccine; BCG: Bacillus Calmette-Guérin Vaccine; HBV: Hepatitis B Vaccine; TDAP: Tetanus-Diphtheria-Acelluar Pertussis Vaccine; IPV: Inactivated Polio Vaccine; HPV: Human Papillioma Virus Vaccine; HAV: Hepatitis A Vaccine; AQP4- IgG: Aquaporin-4 Antibodies; NMO: Neuro Myelitis Optica; HIV: Human Immunodeficiency Virus; CSF: Cerebrospinal Fluid; IVIG: Intravenous Immunoglobulins; MMF: Mycophenolate Mofetil.
Table 1: Cases of vaccination-related optic neuritis reported in the medical literature.
Introduction of immunization programs is undoubtedly one of the greatest achievements in the history of medicine. Vaccinations have profoundly changed the spectrum of diseases affecting the human population and contributed to eradication or large reduction of many infectious disease cases (smallpox, poliomyelitis, and measles). However, nowadays vaccines are progressively becoming the victim of their own success, and many issues regarding their safety raise concerns. Neurological complications following vaccinations, although rare, are well-described phenomenon. The earliest descriptions of neurological complications after vaccinations comes from a report of a neuroparalytic syndrome after Pasteur’s rabies immunization in 1889 [29] and since then cases of seizures, GBS, peripheral neuropathy, encephalopathy and others have been recognized [30,31]. In the recent years the association of vaccines with autoimmune diseases is emerging as the new area of research. As autoimmune phenomena are usually documented within weeks following the vaccination, the plausible causal relationship between vaccination and development of autoimmunity is difficult to delineate and therefore a subject to reporting bias [32].
Moreover, as the overall risk of vaccination-related serious side effect, including autoimmune reactions, is immensely low (i.e. autoimmune mediated demyelinating disease-0.1%), collecting a representative study group for further research is unavailable.
Nevertheless, there are some examples in which the dubious link between vaccines and autoimmunity seems more than logical. In 1976 an outbreak of GBS followed immunization with the “swine flu” vaccine [33]. Similar causal relationship has been shown in transverse myelitis (after oral polio vaccine), in arthritis (following DTP and MMR vaccine) and in autoimmune thrombocytopenia (after MMR vaccine) [34]. In addition, a number of animal models enabled a better way of studying the cause and effect relationship between vaccines and autoimmunity. Vaccination of female mice with aluminum adjuvants and HPV vaccine led to behavioral abnormalities [35], immunization of dogs induced the production of 9 different autoantibodies including lupus-associated ones [36] and vaccination of diabetic prone newborn animals was associated with an increased occurrence of diabetes mellitus [37]. Intra-peritoneal immunization of salmon fish with vaccines embedded in oil-adjuvants also induced autoantibodies and the outbreak of granulomatous disease of the liver and peritoneum and immune mediated glomerulonephritis [38]. Specifically, regarding vaccination-induced autoimmunity of the CNS, application of unpurified rabies vaccine (which contained fragments of myelin with antigenic properties) was shown to induce encephalomyelitis. [31,39].
The exact pathophysiologic mechanism of post-vaccination optic neuritis is not clearly defined. It could be triggered by a common denominator such as the adjuvant [40] used in the vaccine to enhance the antigen-specific immune response. This phenomenon is well described under the umbrella term “autoimmune/ inflammatory syndrome induced by adjuvants” (ASIA) [24], which also includes the Gulf War syndrome, macrophagic myofascitis, siliconosis and others. Aluminium salts are the most widely used adjuvants in human vaccines [41]. There are many hypotheses concerning the booster mechanism of aluminum on the immune responses, including forming nodules slowly releasing antigen (“depot theory”) [42], role of the NACHT, LRR and PYD domains-containing protein-3 (Nlrp3) inflammasome, increased interleukin-4 production [43] release of uric acid [44] and activation of the Syk-PI3 kinase pathway [45-47]. Notably, the vast majority of adverse manifestations experimentally triggered by aluminum in animal models or associated with administration of adjuvanted vaccines in humans are neurological and neuropsychiatric. Interestingly, aluminum particles have a capacity to cross the blood-brain and blood-cerebrospinal fluid barriers and incite immunoinflammatory response in neural tissues [48,49]. Moreover, as the size of aluminum-adsorbed antigen complexes is greater than the molecular weight cut-off of the glomerulus, the effective clearance of aluminum from the body is impaired, resulting in accumulation of this particle in the organism [50]. In our analysis we have observed that vaccines containing aluminum were associated with delayed ON onset in comparison to aluminum – free injections, what may be explained by the delayed clearance of this particle after the vaccination. Interestingly, isolated ON was most frequently reported after influenza vaccine, which is aluminum-free, whereas vaccines containing this element were the leading causes of further demyelination of CNS (MS, NMOSD), what may support the theory of this adjuvant higher neurotoxicity. Nevertheless, the impact of MS cases associated with HBV vaccine increased reporting in VAERS due to increased media attention and public awareness on that topic influencing our results cannot be excluded.
Molecular mimicry is another theory explaining the enigmatic link between vaccines and autoimmunity, described in cases of HBV vaccine (mimicry with myelin basic protein 1) [51] and HPV vaccine (L1 HPV peptide homology to proteins associated with SLE [52].
The concept of molecular mimicry is based on the structural similarity between a pathogen or metabolite and self-structures. The similarity could be expressed as shared amino acid sequences or similar conformational structure between a pathogen and selfantigen [53]. The mimicry between Campylobacter jejuni and GM1 ganglioside antigen, one of the targets of the autoantibodies commonly present in GBS patients, served as a flagship example of this interplay. Interestingly, the association between GBS and Campylobacter jejuni infection was most staggeringly reported during the mass influenza immunization in the United States, prompted by ”swine flu” outbreak, as there was a significant increase in GBS incidence among the vaccinated population. Years later it was demonstrated that mice immunized with the vaccine developed antiganglioside antibodies (anti-GM1), thus supporting the hypothesis that molecular mimicry was the link between vaccine and disease [54]. Nevertheless, the study conducted by Nachamkin et al. in 2008 [55] shed a new light on that topic. In this experiment the vaccines used in 1976, as long as influenza vaccines from 1991-1992 and 2004-2005 were tested for presence of C. jejuni and the ability to induce anti-GM1 antibodies production in mice. Intriguingly, it was observed that none of the tested vaccines contained C. jejuni, although all of them contained glycolipid-like molecules and induced anti-GM1 antibodies in mice. This effect was reported as related to influenza virus hemagglutinin ability to induce anti-GM1. In our analysis we have observed that vaccination against influenza was the leading cause of isolated ON. Interestingly, the association of isolated ON with the presence of antiganglioside antibodies (GQ1b/GT1a) was reported in the literature, in coherence with the fact that the optic nerve is the second most concentrated area of these gangliosides [56]. Regrettably, in none of the case reports qualified to analysis in this study the presence of antiganglioside antibodies was tested, so further conclusions cannot be formulated, although this issue seems a promising area for future research [57-97].
Although it is difficult to establish a causal relationship between vaccination and optic neuritis, we nevertheless believe it is important to make such cases known, both to contribute to the continued development of safe and efficacious methods of vaccination, as well as to provoke further investigation into the pathogenesis of ON. Physicians should be aware of this rare adverse effect of vaccination and performing full antibody profile screening (anti-AQP4, anti- MOG, anti-ganglioside, anti-phosphatidylcholine) when patient presents symptoms of ON should be encouraged.
JR declares no conflict of interest relevant to this study.
Citation: Roszkiewicz J, Shoenfeld Y (2021) Vaccines and Optic Neuritis: Consequence or Coincidence? Immunome Res. 17:7467.
Received: 11-Dec-2020 Accepted: 25-Dec-2020 Published: 01-Jan-2021 , DOI: 10.35248/1745-7580.21.s4.7467
Copyright: © 2021 Roszkiewicz J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.