
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2023)Volume 6, Issue 3
Diabetic Nephropathy (DN), one of the common chronic complications of diabetes, is the leading cause of end-stage renal disease. vaccarin, a highly active chinese medicinal monomer isolated from vaccariae semen, confers protective effects against Type 2 Diabetes Mellitus (T2DM). However, the effects of vaccarin on kidney injury in DN remain unclear. Our study showed that vaccarin ameliorated renal dysfunction and histological damage in diabetic mice through inhibiting renal fibrosis, overproduction of inflammation cytokine and Reactive Oxygen Species (ROS). Additionally, vaccarin treatment significantly suppressed the process of Epithelial-to-Mesenchymal Transition (EMT), a key step for renal fibrosis, in High Glucose (HG)-induced Hexokinase 2 (HK-2) cells. Mechanistically, the network pharmacology analysis and molecular docking revealed that Epidermal Growth Factor Receptor (EGFR) may be the potential target of vaccarin. In support, the phosphorylated levels of EGFR and its downstream mediator Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) were abrogated by vaccarin in diabetic kidneys and HG-treated HK-2 cells. Blockade of either EGFR or ERK1/2 showed similar renal benefits as vaccarin. In conclusion, our results reveal that vaccarin attenuates diabetic renal damage via inactivation of EGFR signaling.
Diabetic nephropathy; Vaccarin; Hexokinase 2 cells; Epidermal growth factor receptor
DN: Diabetic Nephropathy; T2DM: Type 2 Diabetes Mellitus; ROS: Reactive Oxygen Species; EMT: Epithelial-to-Mesenchymal Transition; HG: High Glucose; HK-2: Hexokinase 2; EGFR: Epidermal Growth Factor Receptor; ERK: Extracellular signal-Regulated Kinase; ECM: Extracellular Matrix; TGF: Transforming Growth Factor; ErbB: Erythroblastic Leukemia Viral Oncogene Homologue; H2O2:Hydrogen Peroxide; ox-LDL: Oxidized Low-Density Lipoprotein; EndMT: Endothelial-Mesenchymal Transition; HUVECs: Umbilical Vein Endothelial Cells; MAPK: Mitogen-Activated Protein Kinase; Scr: Serum creatinine; BUN: Blood Urea Nitrogen; α-SMA: α-Smooth Muscle Actin; HFD: High-Fat Diet; STZ: Streptozocin; i.p:Intra-Peritoneal injection; VAC: Vaccarin Control Group; FBG: Fast Blood Glucose; H&E: Hematoxylin and Eosin; PAS: Periodic Acid-Schiff; BSA: Body Surface Area; HRP: Horseradish Peroxidase; DAB: Diaminobenzidine; RNA: Ribonucleic Acid; PCR: Polymerase Chain Reaction; RIPA: Ristocetin-Induced Platelet Aggregation; BCA: Body Composition Analysis; SDS-PAGE: Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis; PVDF: Polyvinylidene Fluoride; TBS: Tris-Buffered Saline; TBST: TBS Tween-20; IgG: Immunoglobulin G; CO2: Carbondioxide; ANOVA: Analysis of Variance; IHC: Immunohistochemistry; mRNA: Messenger RNA; E-cadherin: Epithelial Cadherin; RT-PCR: Quantitative Real Time-PCR; IL-1β: Interleukin-1β; VCAM-1: Vascular Cell Adhesion Molecule-1; COX-2: Cyclooxygenase-2; DHE: Dihydroethidium
Diabetic Nephropathy (DN), one of the most serious micro vascular complications of diabetes, is taken as a leading cause of end-stage renal disease [1,2]. According to the latest research, it is estimated that 550 million people will suffer from diabetes all over the world by 2030, and approximately 50% of patients with type 2 diabetes will develop into DN [3,4]. DN is characterized by proteinuria, excessive mesangial matrix formation and renal fibrosis [5,6]. Studies have shown that renal fibrosis is the main pathological event in the development of DN [7]. Renal fibrosis is usually characterized by the deposition of Extracellular Matrix (ECM), including collagen I, in the renal tubulointerstitium [8]. Renal tubular epithelial cells have been damaged in the early stage of DN [9]. Long-term exposure to hyperglycemia leads to their tubular structure to renal interstitial structure known as EMT. Once the process of EMT happened, ECM will be dispersed around tubular structures, which evolved into the accumulation of ECM in the tubulointerstitial, causing irreversible damage to the renal structure [10,11]. Accumulating evidence implicates that Transforming Growth Factor-β1 (TGF-β1) constitutes an important fibrogenic cytokine in the development of DN by activating Smad signaling pathway [12,13].
EGFR, a member of the Erythroblastic Leukemia Viral Oncogene Homologue (ErbB) family of receptor tyrosine kinases, is generally expressed in renal epithelial cells [14,15]. Ligand binding to EGFR leads to activation of the intrinsic kinase domain at Y1173 [14,16]. In addition, EGFR can be phosphorylated at Y845 by non-ligand pathway mediated by oxidative stress [17]. Recent studies have shown that EGFR is a pivotal mediator for renal fibrosis [18,19]. HG-mediated EGFR phosphorylation and ERK activation, consequently promotes TGF-β expression and induces renal fibrosis [17]. Despite the growing understanding of diabetic renal pathology, DN remains a leading cause of morbidity and mortality in humans. Therefore, it is imperative to develop for novel drugs to treat DN.
Vaccarin, a natural flavonoid glycoside [20], exhibits a broad of pharmacological effects, such as anti-oxidation, anti-inflammation and anti-hyperglycemic actions [21-23]. Our group has shown that vaccarin alleviates HG- and Hydrogen Peroxide (H2O2)-induced endothelial cell injury via inhibiting the notch signaling [24,25]. Besides, vaccarin treatment ameliorates nephropathy and cardiovascular remodeling in hypertensive rats [26]. Recently, it has been demonstrated mechanism of vaccarin in DN. With the aid of network pharmacology and molecular docking, EGFR was identified as a potential target of vaccarin. Whether and how EGFR mediated the renal benefits of vaccarin was largely unknown. The present study thus explored the potential effects of vaccarin on DN at both animal and cell levels, and investigated whether vaccarin ameliorated renal injury by acting on the EGFR signaling pathway in T2DM. That vaccarin improves the disorder of glucose and lipid metabolism in T2DM mice [27,28]. Moreover, vaccarin prevents oxidized Low-Density Lipoprotein (ox-LDL)-induced Endothelial-Mesenchymal Transition (EndMT) in Human Umbilical Vein Endothelial Cells (HUVECs) via suppressing ROS/P38 Mitogen-Activated Protein Kinase (MAPK) signaling pathway [20]. Overall, vaccarin is likely beneficial for attenuating cardiovascular and metabolic disorders. However, little is known regarding the role and underlying mechanism of vaccarin in DN. With the aid of network pharmacology and molecular docking, EGFR was identified as a potential target of vaccarin. Whether and how EGFR mediated the renal benefits of vaccarin was largely unknown. The present study thus explored the potential effects of vaccarin on DN at both animal and cell levels, and investigated whether vaccarin ameliorated renal injury by acting on the EGFR signaling pathway in T2DM.
Reagents and chemicals
Vaccarin was purchased from shifeng technology (shanghai, China). D-glucose was bought from sigma chemical Co. (st louis, Missouri, USA). Kits for Serum creatinine (Scr), Blood Urea Nitrogen (BUN) and urine protein were procured from jiancheng bioengineering institute (nanjing, China). Antibodies against β-actin, α-Smooth Muscle Actin (α-SMA), E-cadherin and Phosphorylated-ERK were acquired from cell signaling technology (danvers, Massachusetts, USA). Antibodies against collagen I, TGF-β1, T-EGFR, P-EGFR(Y845), P-EGFR(Y1173) and T-ERK from abcam (cambridge, Massachusetts. USA).
Experimental animals
Male C57BL/6J mice aged 6-8 weeks were purchased from the model animal research center of nanjing university (nanjing, China). All experiments were approved by institutional animal’s care and use committee at jiangnan university (document number for animal use approval: JN.No20200710c0600131(174)). Animals were caged in a light-dark cycle of 12 hours, temperature and humidity-controlled room and given free access to chow and tap water.
Mice model establishment
T2DM was experimentally induced in mice via a High-Fat Diet (HFD)/Streptozocin (STZ) regimen as we previously demonstrated [20,27]. After one week of acclimatization, 40 mice were randomly divided into four groups. The normal control group were given standard diet. Group received vaccarin daily (1 mg/kg, i.p.) for 8 weeks was served as Vaccarin Control Group (VAC). While the other mice were given HFD (21.8 kJ/g, 60% fat, D12492). After feeding for 4 weeks, the HFD mice were received a single dose of STZ i.p. (120 mg/kg, 10 mM precooled citrate buffer, pH 4.0 dissolved) after fasting for 12 h. Mice with fasting plasma glucose more than 11.1 mmol/L were considered to be diabetic [29]. Thereafter, T2DM mice were randomly allocated to two groups, model group (DN) and vaccarin-treated DN group (DN+VAC). DN+VAC group was given vaccarin (1 mg/kg, i.p.) every day for 8 weeks. Mice in DN group and (DN+VAC) group were maintained on HFD until sacrificed.
Assessment of blood glucose level, albuminuria, blood urea nitrogen and serum creatinine
Fast Blood Glucose (FBG) was measured using an AccuChek glucose meter weekly. At the end of experiment, metabolic cages were used to collect 24 h urine for albuminuria analysis. The blood was collected to separate serum samples, and the serum levels of BUN and Scr were measured according to the manufacturer’s recommendations [30].
Sample collection and morphological observations
Mice were sacrificed after anaesthesia. Both kidneys were removed and weighed for renal/body weight index calculation. Kidney was fixed with 4% paraformaldehyde and paraffin kidney sections were cut at 5 μm. The renal histological changes were assessed by Haematoxylin and Eosin (H&E), Periodic Acid-Schiff (PAS). Masson staining was used to evaluate the renal fibrosis. Images were captured by the panoramic scan (3 dhistech Ltd., budapest, hungary).
Immunohistochemistry assay
Renal sections were de-paraffinized, hydrated and underwent antigen retrieval (pH=6 citrate buffer, at 95°C for 15 min). Thereafter, sections were incubated with H2O2 (3%) for removing endogenous peroxidase activity before blocking with 5% Body Surface Area (BSA) for 60 min. Then, sections were probed with primary antibodies against collagen Ⅰ, α-SMA and E-cadherin overnight at 4°C. Subsequently, the sections were subject to Horseradish Peroxidase (HRP)-coupled secondary antibodies for 60 min at room temperature. Finally, 3,3-Diaminobenzidine (DAB) was applied for staining. The images were observed under the panoramic scan.
Quantitative real time-PCR
Total RNA was isolated from tissues and cells using trizol reagent (cwbio, beijing, China) following the manufacturer’s instructions. Equal RNA was used hiscriptq RT supermix (vazyme, nanjing, China) for reverse transcription and quantitative real time-PCR was performed using chamQTM SYBR® qPCR master mix (vazyme, nanjing, China). 2-ΔΔCT method was used to show relative gene expression levels [31].
Western blotting
Total protein was isolated using RIPA lysis buffer (cwbio, taizhou China). The protein concentration was assayed by BCA kit (beyotime biotechnology, shanghai, China). Proteins (20 μg) were loaded onto Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gels, and then transferred to the Polyvinylidene Fluoride (PVDF) membranes. The membranes were blocked with defatted milk in Tris-Buffered Saline (TBS) with 0.1% Tween-20 (TBST) for 1h and incubated with the primary antibodies at 4°C overnight. After washing with TBST, the membranes were incubated with a 1:2000 dilution of HRP conjugated anti-rabbit IgG antibody (CWBIO, taizhou China). The blots were visualized by a chemiluminescence detection system (millipore darmstadt, Germany) and semi-quantified by image j (national institutes of health, bethesda, Maryland, USA).
Cell culture and treatments
HK-2 cells (a human kidney tubular epithelial cell line, fudan ibs cell center, shanghai, China) were cultured in low glucose dulbecco's modified eagle medium medium (5.6 mM glucose, gibco, carlsbad, California, USA) supplemented with 10% fasting blood sugar and 1% penicillin/streptomycin (gibco, carlsbad, California, USA) in a 5% CO2 incubator. The cells were treated with 5 μM vaccarin for 12 h before HG (35 mM) for 48 h in the following experiments.
Databases analysis
Genes related to DN and diabetic kidney disease were screened from the drug bank database (https://go.drugbank.com/) and gene cards database (https://www.genecards.org/). The predicting targets of vaccarin were searched from pharmmapper database (http://www.lilab-ecust.cn/pharmmapper/). The david database (https://david.ncifcrf.gov/) was used to predict the biological processes. High-confidence proteins of the protein-protein interaction network was constructed by the string database (https://string-db.org/) and visualized by cytoscape.
Molecular docking of EGFR and vaccarin
The 3D structure of EGFR was downloaded from rcsb Protein Data Bank (PDB) (http://www.rcsb.org/); the molecular structure of vaccarin was provided from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Autodock (http://autodock.scripps.edu/) was used to dock EGFR and vaccarin based on network pharmacology. Binding energy was used as docking score to predict binding strength between the key target and the drug.
Measurement of oxidative stress markers
The superoxide anion production in cells was detected by Dihydroethidium (DHE, 10 Μm) staining in a dark environment for 30 min at 37°C. The images were captured under a fluorescence microscope (zeiss, Germany). Besides, the levels of Malondialdehyde and the activity of glutathione in the diabetic kidney tissue were determined according to the manufacturer’s recommendations [32].
Statistical analysis
Data were represented as mean ± SEM. Statistical comparison was performed by one-way analysis of variance (one-way ANOVA) using Graphpad prism 8 software (california, USA). P<0.05 was considered statistically significant.
Vaccarin alleviates renal dysfunction in diabetic mice
Firstly, we assessed the therapeutic effects of vaccarin on renal damage induced by diabetes in vivo. Compared with control mice, the FBG level was significantly elevated in DN mice and reversed by vaccarin treatment (Figure 1A). Moreover, the levels of urine albumin, BUN and Scr, as well as renal/body weight index were significantly higher in DN group than those in control group. However, treatment of vaccarin alleviated these parameters in diabetic mice (Figures 1B-1E). Morphological analysis from H&E staining and PAS staining demonstrated that the glomeruli was hypertrophic in diabetic mice, which was obviously ameliorated by vaccarin (Figures 1F and1G). Of note, there were no significant changes on renal morphology and renal function tests in normal mice and DN mice administrated with vaccarin. These findings indicated that vaccarin protected kidney from damage in T2DM mice.
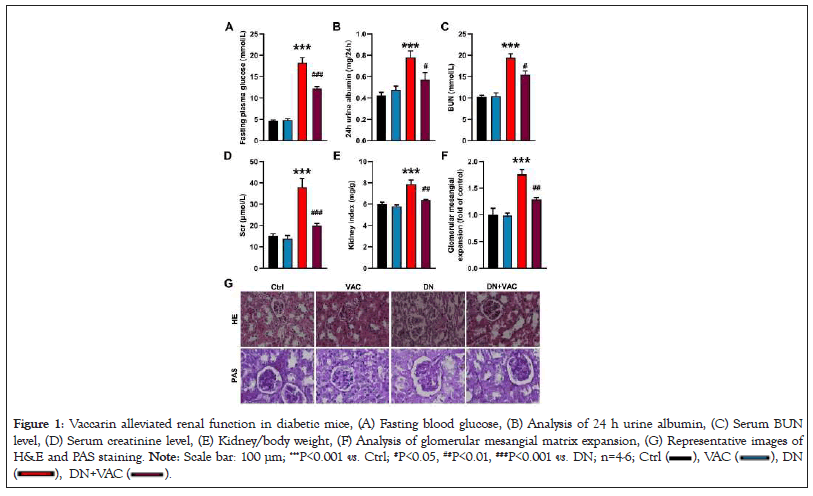
Figure 1: Vaccarin alleviated renal function in diabetic mice, (A) Fasting blood glucose, (B) Analysis of 24 h urine albumin, (C) Serum BUN
level, (D) Serum creatinine level, (E) Kidney/body weight, (F) Analysis of glomerular mesangial matrix expansion, (G) Representative images of
H&E and PAS staining. Note: Scale bar: 100 μm; ***P<0.001 vs. Ctrl; #P<0.05, ##P<0.01, ###P<0.001 vs. DN; n=4-6; Ctrl 
Vaccarin attenuates renal fibrosis in diabetic mice
Renal fibrosis plays an important role in the progression of DN [33]. Accumulation of ECM is the primary feature of renal fibrosis [34]. Collagen I is one of the essential components in the process of ECM [35]. Masson staining demonstrated that vaccarin attenuated renal fibrosis in diabetic mice (Figures 2A and 2B). Immunohistochemistry (IHC) results further confirmed that vaccarin reduced renal fibrosis in diabetic mice, as manifested by lower collagen I immuno-positive signals (Figures 2A and 2C). In similarity, increased collagen I mRNA level were detected in diabetic mice compared with control group. However, treatment with vaccarin substantially reversed these abnormalities (Figure 2D). A myriad of evidence implicates the role of EMT in the accumulation of ECM [36,37]. The process of EMT is accompanied by upregulation of fibroblast markers, such as α-SMA, and downregulation of epithelial indices, including Epithelial Cadherin (E-cadherin) [38]. IHC results indicated that vaccarin diminished EMT process in T2DM mice, as indicated by higher E-cadherin and lower α-SMA immuno-positive signals (Figures 2A and 2C). Consistent with the IHC results, vaccarin was found to downregulate α-SMA mRNA level (Figure 2F) and upregulate E-cadherin mRNA level (Figure 2G) in diabetic mice. TGF-β1 may play a key role in the processes of EMT under diabetic conditions [39,40], RT-PCR results demonstrated that upregulation of TGF-β1 in diabetic mice was attenuated by vaccarin (Figure 2E). These findings indicated that vaccarin might ameliorate diabetic renal fibrosis, especially by arresting EMT progression of renal tubular cells.
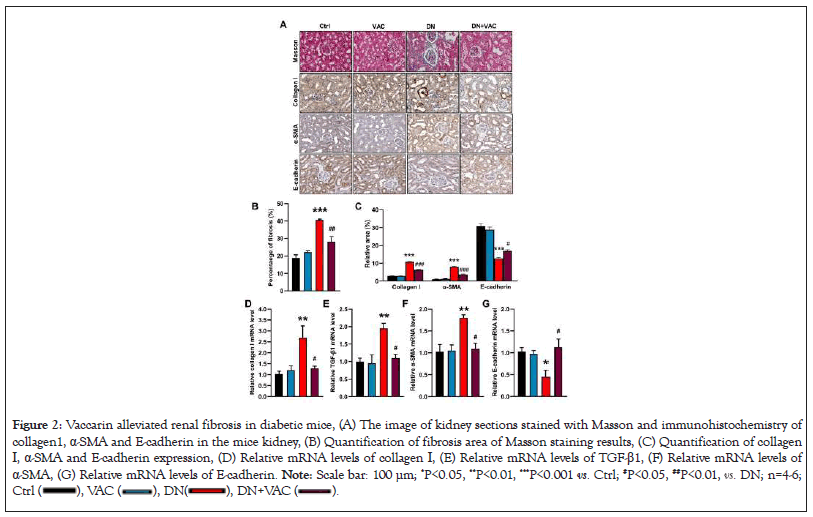
Figure 2: Vaccarin alleviated renal fibrosis in diabetic mice, (A) The image of kidney sections stained with Masson and immunohistochemistry of
collagen1, α-SMA and E-cadherin in the mice kidney, (B) Quantification of fibrosis area of Masson staining results, (C) Quantification of collagen
I, α-SMA and E-cadherin expression, (D) Relative mRNA levels of collagen Ⅰ, (E) Relative mRNA levels of TGF-β1, (F) Relative mRNA levels of
α-SMA, (G) Relative mRNA levels of E-cadherin. Note: Scale bar: 100 μm; *P<0.05, **P<0.01, ***P<0.001 vs. Ctrl; #P<0.05, ##P<0.01, . DN; n=4-6; 
Vaccarin attenuates renal inflammation and oxidative stress in diabetic mice
Abnormal inflammatory response and oxidative damage are the driving factors of DN [32]. Hyperglycemia resulted in strong inflammatory responses in the kidneys as evidence by the increased mRNA levels of IL-1β, VCAM-1, COX-2 and p65-NFkB, whereas these effects were effectively reversed by vaccarin (Figures 3A-3D). Also, we found increased renal muscular dystrophy association content, but decreased renal activities of glutathione peroxidase in diabetic mice, whereas these aberrant changes were blunted by treatment with vaccarin (Figures 3E and 3F). Moreover, the results from DHE staining and dichloro-dihydro-fluorescein diacetate staining in the kidney tissues showed the decreased renal oxidative stress in diabetic mice when treated with vaccarin (Figures 3G-3I). These above results hinted that vaccarin alleviated inflammatory response and reduced ROS production in diabetic kidneys.
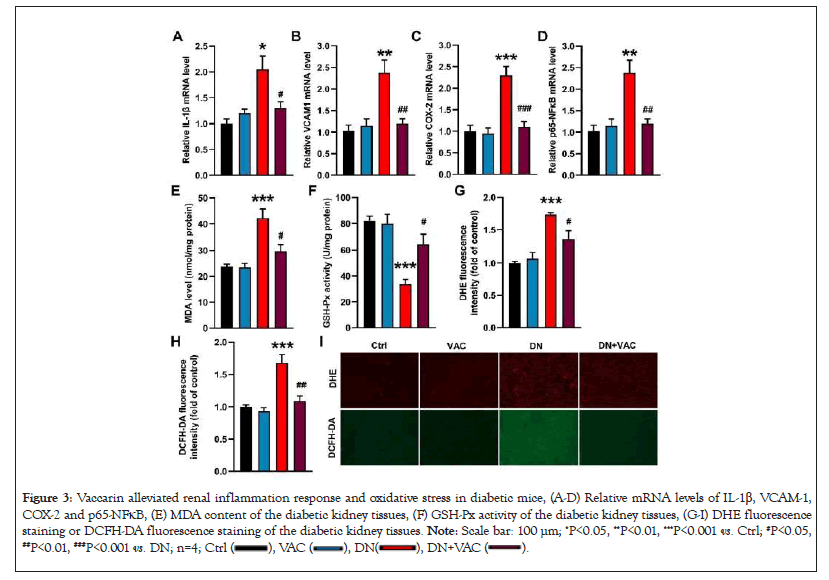
Figure 3: Vaccarin alleviated renal inflammation response and oxidative stress in diabetic mice, (A-D) Relative mRNA levels of IL-1β, VCAM-1,
COX-2 and p65-NFκB, (E) MDA content of the diabetic kidney tissues, (F) GSH-Px activity of the diabetic kidney tissues, (G-I) DHE fluorescence
staining or DCFH-DA fluorescence staining of the diabetic kidney tissues. Note: Scale bar: 100 μm; *P<0.05, **P<0.01, ***P<0.001 vs. Ctrl; #P<0.05, ##P<0.01, ###P<0.001 vs. DN; n=4; 
Vaccarin attenuates HG-induced EMT in HK-2 cells
We further moved on to assess the potential effects of vaccarin on HG-induced HK-2 cells. The results of western blotting and RT-PCR revealed that pretreatment with vaccarin substantially reversed higher collagen I, α-SMA and TGF-β1 levels, but lower E-cadherin level in HG-challenged HK-2 cells (Figures 4A-4E). Additionally, as expected, the control cells treated with vaccarin showed no changes in ECM deposition and progress of EMT. These results indicated that the process of EMT is involved in vaccarin-mediated renal protective effects.
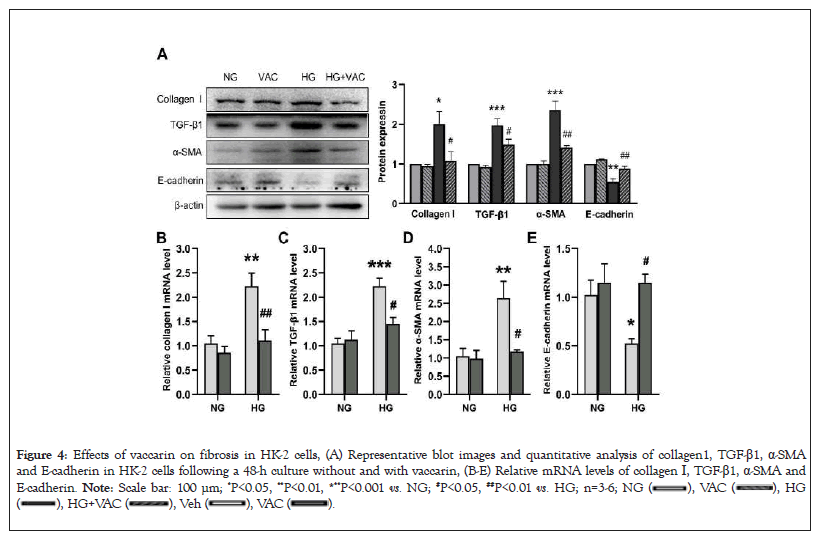
Figure 4: Effects of vaccarin on fibrosis in HK-2 cells, (A) Representative blot images and quantitative analysis of collagen1, TGF-β1, α-SMA
and E-cadherin in HK-2 cells following a 48-h culture without and with vaccarin, (B-E) Relative mRNA levels of collagen I, TGF-β1, α-SMA and
E-cadherin. Note: Scale bar: 100 μm; *P<0.05, **P<0.01, ***P<0.001 vs. NG; #P<0.05, ##P<0.01 vs. HG; n=3-6; 
Vaccarin attenuates HG-induced inflammation response and oxidative injury in HK-2 cells
Next, we determined whether vaccarin could ameliorate HG-stimulated inflammation response and oxidative stress in HK-2 cells. The mRNA levels of inflammatory factor markers, including IL-1β, VCAM-1, COX-2 and p65-NFkB were remarkably enhanced when HK-2 cells were exposed to HG, effects that were strikingly blocked by vaccarin (Figures 5A-5D). Besides, upon exposure to HG, the overproduction of intracellular ROS was substantially abrogated by preincubating with vaccarin in HK-2 cells (Figures 5E-5G).
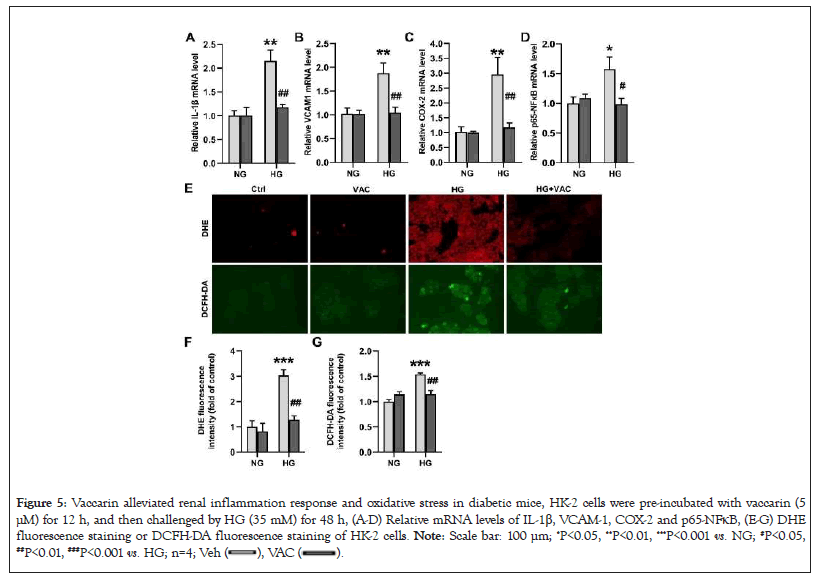
Figure 5: Vaccarin alleviated renal inflammation response and oxidative stress in diabetic mice, HK-2 cells were pre-incubated with vaccarin (5
μM) for 12 h, and then challenged by HG (35 mM) for 48 h, (A-D) Relative mRNA levels of IL-1β, VCAM-1, COX-2 and p65-NFκB, (E-G) DHE
fluorescence staining or DCFH-DA fluorescence staining of HK-2 cells. Note: Scale bar: 100 μm; *P<0.05, **P<0.01, ***P<0.001 vs. NG; #P<0.05, ##P<0.01, ###P<0.001 vs. HG; n=4; 
Vaccarin ameliorates renal fibrosis by inhibiting EGFR signaling pathway
To further explore the underlying mechanism of vaccarin in alleviating renal fibrotic lesions of diabetic mice, we conducted a network pharmacological analysis. 107 intersection gene of vaccarin and DN were identified (Figure 6A). A protein-protein interaction network of common targets was built [41]. EGFR was obviously at the center of the network (Figure 6B). Biological process is a significant aspect of gene ontology enrichment analysis [31], we found that ERBB2 signaling pathway, epidermal growth factor receptor signaling pathway, positive regulation of smooth muscle cell proliferation and negative regulation of apoptotic process significantly enriched (Figure 6C), which were verified to contribute to renal fibrosis [34,42,43]. Molecular docking demonstrated a direct interaction between vaccarin and EGFR with binding energy of -8.8 Kcal/mol (Figure 6D).
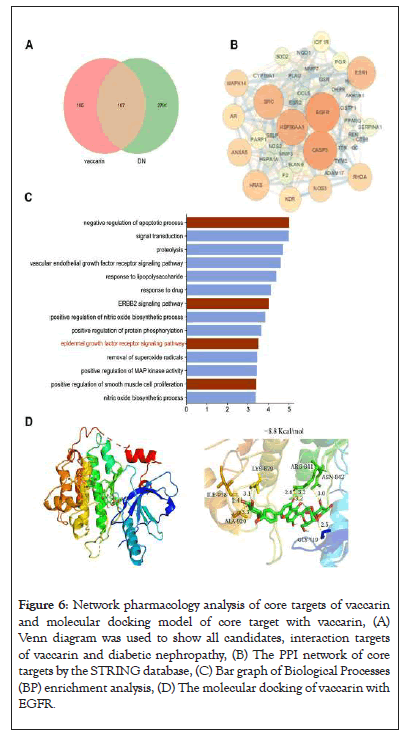
Figure 6: Network pharmacology analysis of core targets of vaccarin and molecular docking model of core target with vaccarin, (A) Venn diagram was used to show all candidates, interaction targets of vaccarin and diabetic nephropathy, (B) The PPI network of core targets by the STRING database, (C) Bar graph of Biological Processes (BP) enrichment analysis, (D) The molecular docking of vaccarin with EGFR.
It is highly probably that EGFR is the key target of renal injury based on bioinformatics and molecular docking. More importantly, EGFR is involved in DN through the activation of ERK1/2 signaling pathway [15]. Thereafter, we sought to determine whether vaccarin ameliorated renal injury by acting on the EGFR/ERK signaling pathway in T2DM. Our results showed that the phosphorylated levels of EGFR (Y845, Y1173) and ERK1/2 in the kidney were augmented, and this effect was counteracted by treatment with vaccarin (Figures 7A, 7C-7E). Besides, the HG-induced upregulation of EGFR/ERK phosphorylation in HK-2 cells were also attenuated by vaccarin treatment, as demonstrated by western blotting (Figures 7B, 7F-7H). Additional experiments were performed to test whether the EGFR/ERK signaling contributed to hyperglycemia-induced renal damage, therefore, HK-2 cells were treated with or without EGFR inhibitor AG1478 or ERK inhibitor U0126 in the context of HG. We found AG1478 or U0126 significantly suppressed HG-induced fibrosis (Figures 8A-8D), inflammation (Figures 8E-8H) and oxidative stress (Figure 8I).
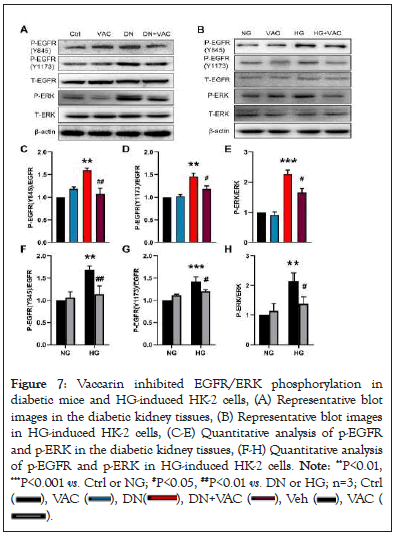
Figure 7: Vaccarin inhibited EGFR/ERK phosphorylation in
diabetic mice and HG-induced HK-2 cells, (A) Representative blot
images in the diabetic kidney tissues, (B) Representative blot images
in HG-induced HK-2 cells, (C-E) Quantitative analysis of p-EGFR
and p-ERK in the diabetic kidney tissues, (F-H) Quantitative analysis
of p-EGFR and p-ERK in HG-induced HK-2 cells. Note: **P<0.01,
***P<0.001 vs. Ctrl or NG; #P<0.05, ##P<0.01 vs. DN or HG; n=3; Ctrl 
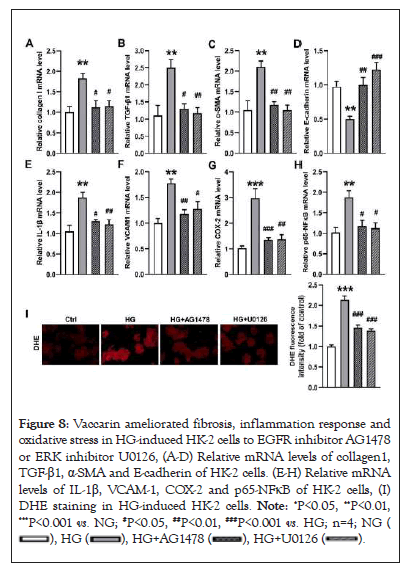
Figure 8: Vaccarin ameliorated fibrosis, inflammation response and
oxidative stress in HG-induced HK-2 cells to EGFR inhibitor AG1478
or ERK inhibitor U0126, (A-D) Relative mRNA levels of collagen1,
TGF-β1, α-SMA and E-cadherin of HK-2 cells. (E-H) Relative mRNA
levels of IL-1β, VCAM-1, COX-2 and p65-NFκB of HK-2 cells, (I)
DHE staining in HG-induced HK-2 cells. Note: *P<0.05, **P<0.01,
***P<0.001 vs. NG; #P<0.05, ##P<0.01, ###P<0.001 vs. HG; n=4; NG ( 
In the present study, we found that administration of vaccarin efficiently attenuated diabetic renal injury via preserving renal function, improving biochemical parameters, ameliorating morphological abnormalities and antagonizing renal fibrosis in mice. We also observed that vaccarin inhibited HG-induced HK-2 cells fibrosis, inflammation response and oxidative stress via inactivation of EGFR/ERK signaling. In summary, our study suggested that vaccarin may serve as a new potential approach for the treatment of DN.
DN is a severe renal microvascular complication, arising as a consequence of persistent hyperglycemia [44]. Uncontrolled hyperglycemia causes renal structural damage, including glomerular lesions, microalbuminuria, mesangial expansion and interstitial fibrosis [4,44]. In this study, vaccarin was found to decrease the level of blood glucose of diabetic mice. In addition, we found that vaccarin improved diabetic renal dysfunction by reducing the level of creatinine, BUN, and albuminuria. Histological examination by H&E and PAS staining further suggested the therapeutic effect of vaccarin in DN. In the pathophysiology of DN, renal fibrosis plays an important role [45]. The development of renal fibrosis is related to the increase of fibroblasts and myofibroblasts, which produce ECM in the renal tubular interstitial space [46]. Moreover, hyperglycemia can lead to EMT of renal tubular epithelial cells in DN, and EMT is one of the reasons for myofibroblast activation and synthesis of ECM [47]. Additionally, studies have established that TGF-β1 is a key mediator of renal fibrosis in diabetes [48]. A previous study showed that HG-mediated EGFR phosphorylation and ERK activation, consequently promotes TGFβ expression and induces renal fibrosis [17]. Our present results demonstrated that vaccarin treatment retarded the progression of EMT and diabetic renal fibrosis in vivo and in vitro, as manifested by upregulated E-cadherin, downregulated collagen Ⅰ, α-SMA and TGF-β1. These results indicated that vaccarin ameliorated renal fibrosis in diabetic mice via antagonizing the process of EMT.
Inflammation response and oxidative stress are considered the pathologies of DN [49]. In this study, our results found that vaccarin exhibited an anti-inflammatory effect by inhibiting expressions of inflammatory factors in both cells and kidneys under hyperglycemia conditions. Also, vaccarin alleviated oxidative stress in both diabetic renal tissues and HG-treated HK-2 cells. These results demonstrated that vaccarin may be taken as a therapeutic agent for diabetic kidney disease by inhibiting inflammation response and clearing overproduction of reactive oxygen species.
With the help of network pharmacology and molecular docking, we speculated that vaccarin might be able to ameliorate renal fibrosis and injury through targeting EGFR. Compelling evidence indicates that EGFR plays a necessary role in the process of DN through the activation of ERK1/2 signaling pathway. Our data showed that vaccarin inhibited EGFR phosphorylation and ERK1/2 activation in diabetic kidneys and HG-induced HK-2 cells. Meanwhile, we found that blockade of either EGFR or ERK1/2 ameliorated DN. The current evidence might yield a hypothesis that vaccarin may ameliorate renal fibrosis via inhibiting the EGFR-ERK signaling pathway. Further confirming whether the overexpressed EGFR/ERK signaling pathway counteracted renal protection of vaccarin.
The available results of our study showed that administration of vaccarin could effectively ameliorate renal damage through lowering blood glucose, restoring the biochemical and histological alterations in an experimental model of HFD/STZ-induced T2DM. Moreover, we found that vaccarin preserved diabetes-induced renal injury by inhibiting EMT of renal tubular epithelial cells, inflammation response and oxidative damage via inactivating the EGFR/ERK signaling pathway.
We sincerely thank the support of the experimental public platform of wuxi medical college, jiangnan university. We also thank the laboratory partners accompanied our entire experimental process.
Xuexue Zhu, Xinyao Du, Xinyu Ma performed experiments and wrote the manuscript. Xinyu Meng, Chenyang Zhao, Taiyue Li, Xiaoyi Yu, Xuerui Zhu, Yuanyuan Wen analyzed and processed data. Bao Hou, Weiwei Cai provided technical assistance. Li-Ying Qiu and Fei Xu coordinated and conceived the experiments. All authors corrected and approved the manuscript.
This work was supported by grants from the chinese postdoctoral science fund (Grant no. 2019M661729) and natural science foundation of jiangsu province (Grant no. BK20190597)
The authors declare that they have no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zhu X, Du X, Ma X, Meng X, Zhao C, Li T, et al (2023) Vaccarin Suppresses Diabetic Nephropathy through Inhibiting the EGFR Signaling Pathway. J Clin Chem Lab Med.6:272.
Received: 02-Aug-2023, Manuscript No. JCCLM-23-25925; Editor assigned: 04-Aug-2023, Pre QC No. JCCLM-23-25925 (PQ); Reviewed: 18-Aug-2023, QC No. JCCLM-23-25925; Revised: 25-Aug-2023, Manuscript No. JCCLM-23-25925 (R); Published: 01-Sep-2023 , DOI: 10.35248/2736- 6588.23.6.272
Copyright: © 2023 Zhu X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.