Mycobacterial Diseases
Open Access
ISSN: 2161-1068
ISSN: 2161-1068
Research Article - (2022)Volume 12, Issue 5
Objective: To evaluate the diagnostic efficiency of Bronchoalveolar Lavage Fluid (BALF) for Mycobacterium tuberculosis (MTB) infection using laboratory methods.
Methods: A retrospective study was conducted in patients diagnosed with Active Pulmonary Tuberculosis (APTB) and lacking sputum quality/quantity. Bronchoalveolar Lavage Fluid (BALF) collected during the operation processes of electric bronchoscopy were tested using Ziehl-Neelsen Staining Acid-Fast Bacilli Smear Microscopy (Z-N-AFB-SM), GeneXpert MTB/RIF (Xpert), Loop-Mediated Isothermal Amplification (LAMP), or culturing with BACTEC™ Mycobacterial Growth Indicator Tube™ 960 (MGIT). Chi-square test was used for statistical analysis.
Results: 331 suspected APTB patients were enrolled in this study. 224 of whom were sputum-scarce. 89 were sputum-sufficient and tested negative in both Z-N-AFB-SM and MGIT 960. Among the sputum sufficient patients, BALF-testing confirmed APTB diagnosis in 20.2% (18/89) via Z-N-AFB-SM, and 53.0% (35/89) via MGIT. The total positive rates of BALF testing via four aforementioned methods were 18.2% (57/313), 66.4% (168/253), 61.0% (83/136) and 48.2% respectively. The positive rate of MTB discovered in BALF collected by well-trained respiratory physicians are significantly higher than those collected by anesthetists (χ2=22.48, P<0.01). Total adverse events incidence of BAL was 1.9% (6/313).
Conclusion: BALF has a similar sensitivity and specificity for APTB laboratory diagnosis. It can be used as a complementary diagnostic method for APTB when sputum availability is poor. The proficiency of BALF collection is an important factor affecting the detection results.
Smoking; Hospital policies; Mental illness; Negligence
Mycobacterium tuberculosis (MTB) culture is recognized as the gold- standard of laboratory method for Tuberculosis (TB) infection diagnosis; however, it is difficult to perform when patient’s sputum availability is poor. Tuberculosis (TB) is an ancient infectious disease caused by Bacillus MTB, which usually infect the lung and cause Pulmonary Tuberculosis (PTB). Globally, molecular diagnosis methods are strongly proposed by World Health Organization (WHO) in recent years. Despite the progresses in diagnostics methods, a considerable proportion of TB cases reported were clinically diagnosed rather than laboratory confirmed. In 2016, for example, only 57% of the reported PTB cases were bacteriologically confirmed [1]. In China, unfortunately, it is estimated to be 30% [2], much lower than world average. Therefore, Chinese government have set up the strategy goal of improving PTB pathologic diagnostic rate to over 50% by 2020 in the “13th five-year national plan for tuberculosis prevention and control” [3].Corresponding to this, the Chinese National Pulmonary Tuberculosis (PTB) diagnosis standards were revised on May 1st, 2017. Currently, one of the challenges for the laboratory diagnosis of Pulmonary Tuberculosis (PTB) lies in obtaining quality clinical samples, especially for sputum-scarce patients. However, BronchoAlveolar Lavage (BAL) is a safe and well tolerated sampling technique for bronchoalveolar cell [4], and BronchoAlveolar Lavage Fluid (BALF) obtained during the bronchoscopy is available for Pulmonary Tuberculosis (PTB) laboratory diagnosis [5]. However, published data about Pulmonary Tuberculosis (PTB) diagnosis yield via BALF is limited.
A retrospective evaluation within 16 months (from January 2017 to April 2018) was conducted. Bronchoscopy and BronchoAlveolar Lavage (BAL) were performed for 313 patients suspected of Pulmonary Tuberculosis (PTB) who either had negative sputum smears for Acid-Fast Bacilli (AFB) or could not grow on liquid medium. Patients were diagnosed as APTB if they meet WHO criteria’s [6]. Those with Sputum Smear-Negative Pulmonary Tuberculosis (SSN-PTB) and Sputum Culture-Negative Patients (SCN-PTB) were enrolled.
All patients accepted the following examinations: Electrocardiogram, pulmonary function test, chest CT scanning, prothrombin time test, and routine blood test. If no contraindication for bronchoscopy exists, flexible electric bronchoscopy (Pentax/EB-1530T3, Japan) for trans-nasal or trans oral intubation with 2% lignocaine local injections was conducted after six hours fasting, according to the procedure requirements. 20-30 ml of normal saline was instilled for bronchial washing through the working channel, and then was aspirated into different sterile plastic containers.
BronchoAlveolar Lavage Fluid (BALF) samples were immediately brought to the laboratory, and analyzed with Ziehl-Neelsen Staining Acid-Fast Bacilli Smear Microscopy (Z-N-AFB-SM), Xpert MTB/RIF (Xpert, Cepheid, Sunnyvale, CA, USA), Loop-Mediated Isothermal Amplification (LAMP, Eiken Chemical Co., Ltd, Tokyo Japan), and culture via BACTEC™ Mycobacterial Growth Indicator Tube™ 960 (MGIT, Becton Dickinson Diagnostic Systems, Sparks, MD). Operations and quality controls strictly followed the standard procedure [7-9]. Mycobacterium tuberculosis H37Rv provided by the National Tuberculosis Reference Laboratory (NTRL) (Beijing, China CDC) acted as reference strains.
All patients were tracked for 24-48 hours for adverse events after bronchoscopy, and any symptoms such as pneumothorax, hemorrhage, infection or cardiac arrhythmias were recorded. Bronchoscope was thoroughly cleaned with sterilized water by immersing in 2% glueraldehyde for one hour [4,10]. Anesthetists and well trained respiratory physicians performed the operations. Suspected APTB diagnosis would be confirmed if any one of the laboratory testing mentioned above was positive.
Positive rate of each test was calculated respectively. Chi-square tests for statistical analysis were performed using the SPSS Statistical 20.0 software (Armonk, NY, USA) [11]. A p-value inferior to 0.05 was considered that the difference is significant.
Total 313 suspected APTB patients were enrolled. 71.5% (224/313) were sputum-scarce. 28.5% (89/313) were sputum-sufficient and negative in Z-N-AFB-SM and MGIT 960, and their sputum was collected for further tests. Patient age ranged from 13-80 (mean:34), and 51.1% (160/313) were males. All sputum-scarce patients accepted flexible electronic bronchoscopy and the BronchoAlveolar Lavage Fluid (BALF) were collected (Figure 1).
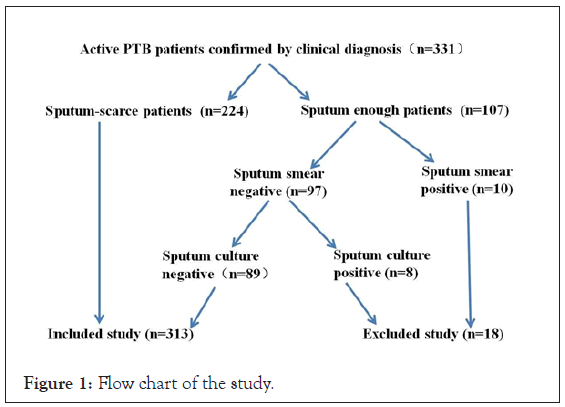
Figure 1: Flow chart of the study.
89 suspected APTB patients who were sputum-sufficient accepted smearing via Z-N staining and liquid culture via MGIT using both samples of sputum and BronchoAlveolar Lavage Fluid (BALF). BALF-testing confirmed 18 suspected patients (20.2%) via Z-NAFB- SM, and 35(53.0%) were confirmed according to liquid culture via MGIT 960 (Table 1). The difference between sputum and BronchoAlveolar Lavage Fluid (BALF) confirmation rate is significant (χ2=20.02,60.69, P<0.01).
| Sample type |
Sample size | Positive (rate) | Sample size | Positive (rate) |
|---|---|---|---|---|
| Z-N-AFB-SM | Liquid culture via MGIT 960 | |||
| Sputum | 89 | 0 | 89 | 0 |
| BALF | 89 | 18 (20.2%) | 66 | 35 (53.0 %) |
Table 1: BALF and sputum for APTB diagnosis via two methods.
All 313 patients were divided into 2 groups, sputum-scarce and sputum-sufficient. BronchoAlveolar Lavage Fluid (BALF) collected from both groups was tested for MTB via different methods. For the sputum-sufficient group, the diagnosis yields were 20.2% (18/89) via Z-N-AFB-SM, 55.1% (49/89) via Xpert, 57.4% (31/54) via LAMP, 53.0% (35/66) via MGIT. For the sputum-scarce group, the diagnosis yields were 17.4% (39/224), 72.5% (119/164), 63.4% (52/82) and 46.9% (105/224) respectively (Table 2). Chi square tests suggest that there were no significant difference between the two groups by using Z-N-AFB-SM, LAMP, and culture (χ2=0.56, 0.49, 0.77, P>0.05), but were significant by using Xpert (χ2=7.92, P<0.05). In our study, the total positive rate using BronchoAlveolar Lavage Fluid (BALF) was18.2% (57/313) by method of Z-N-AFBSM, 66.4% (168/253) by method of Xpert, 61.0% (83/136) by method of LAMP and 48.2% by method of MGIT respectively. The three methods were all significant higher than Z-N-AFB-SM (χ2=119.30, 60.80, 6.57, P 0.01). Xpert had the highest diagnosis yield, the difference between XPERT and MGIT was significant (χ2=25.60, P<0.05). LAMP had the second highest diagnosis yield, the difference between LAMP and MGIT was significant too (χ2=6.57, P<0.05). But the diagnosis yield between Xpert and LAMP were similar (χ2=2.158, P>0.05).
| Sample type | Symptoms | Positive rate (%, Positive/sample size) | |||
|---|---|---|---|---|---|
| Z-N-AFB-SM | Xpert | LAMP | MGIT | ||
| BALF | APTB who were Sputum-sufficient |
20.2 | 55.1 | 57.4 | 53 |
| 18/89 | 49/89 | 31/54 | 35/66 | ||
| APTB who were Sputum-scarce |
17.4 | 72.5 | 63.4 | 46.9 | |
| 39/224 | 119/164 | 52/82 | 105/224 | ||
| X2 | 0.339 | 7.924 | 0.494 | 0.774 | |
| P | 0.561 | 0.005 | 0.482 | 0.379 | |
Table 2: Efficiency of BALF for PTB laboratory diagnosis.
Experienced respiratory physicians who have acquired professional training of electronic bronchoscopy as well as BronchoAlveolar Lavage (BAL) operation and anesthetists who were not well trained operated the BronchoAlveolar Lavage Fluid (BALF) collection. After a serial of laboratory tests mentioned above, the results suggest that the positive rate of Mycobacterium tuberculosis (MTB) discovered in BronchoAlveolar Lavage Fluid (BALF) collected by respiratory physicians are significantly higher than that collected by anesthetists (χ2=22.48, P<0.01). However the total positive rate of Mycobacterium tuberculosis (MTB) discovered in BronchoAlveolar Lavage Fluid (BALF) was 66.5% (Table 3).
| BALF collector | Well-trained for BAL | Sample type | Sample size | Positive | Positive rate (%) |
|---|---|---|---|---|---|
| Respiratory physician | Yes | BALF | 120 | 99 | 82.5 |
| Anesthetist | No | BALF | 193 | 109 | 56.5 |
| Total | BALF | 313 | 208 | 66.5 |
Table 3: Positive rate of BALF collected by different doctor.
Tuberculosis focal spreading outside its original site and metastasizing occurred in 4 APTB patients after bronchoscopy and BronchoAlveolar Lavage (BAL). Another 2 patients suffered fever within 1-2 days after bronchoscopy, and the pulmonary foci increased suddenly. Further sputum culture results identified that pseudomonas aeruginosa caused this secondary-acquired pneumonia. All were cured after receiving standard anti tuberculosis or anti-infection treatments. Total adverse events incidence was 1.9% (6/313).
BronchoAlveolar Lavage Fluid (BALF) has a similar sensitivity and specificity for APTB laboratory diagnosis. So far, culturing of Mycobacterium tuberculosis (MTB) is still deemed as the gold standard for the diagnosis of Tuberculosis (TB) [12]. In 2013, World Health Organization (WHO) made a revision to Tuberculosis (TB) diagnostic criteria that the positive results tested based on molecular methods are equivalent to the positive results tested by traditional bacteriological methods. BronchoAlveolar Lavage (BAL) was initially used to rinse the bronchial tree with saline in 1970. It evolved to a diagnostic tool in India in 1994 [13]. By sampling broncho pulmonary cells and epithelial lining liquid, it can accurately diagnose many infectious diseases and tumor. It is superior to invasive techniques such as needle aspiration biopsy and thoracoscopy, and can be used to diagnose sputum-negative Tuberculosis (TB) at early stage [4]. The positive rate of TB diagnosis by BronchoAlveolar Lavage Fluid (BALF) is 87% [14], and for sputum smear negative PTB it reaches 68.2% [15]. A total of 1214 tuberculosis patients detected by BronchoAlveolar Lavage Fluid (BALF) from 9 studies showed that the sensitivity and specificity of BALF test are 54% (95% CI:48%-59%) and 97% (95% CI:95%- 98%) respectively [16].
Complementary methods for sputum-scarce APTB are GA collection for three consecutive days, which usually requires hospital admissions and BronchoAlveolar Lavage Fluid (BALF) collections, and may not be achievable in every setting [17]. BAL is a valuable method of respiratory tract investigation [18]. In our study, the results disclosed that the BronchoAlveolar Lavage Fluid (BALF) has a significantly higher yield than that of sputum. For those suspected APTB with negative results using sputum both tested by Z-N-AFB-SM and by culture, 20.2% (18/89) and 53.0% (35/66) were confirmed when tested using BronchoAlveolar Lavage Fluid (BALF). However, there was no significant difference between sputum-sufficient and scarce APTB by using BALF, the diagnosis yields of the two groups appeared consistence.
BronchoAlveolar Lavage Fluid (BALF) has different diagnosis yield using different testing methods. In our study, we found AFB stain was positive in 18.2% (N=313) of BALF via Z-N-AFB-SM, and positive liquid culture was 48.2% (N=290) in BALF via MGIT 960. Our positive rate of Z-N-AFB-SM was slightly lower than other study reported, but the positive rate of culture based on liquid medium was significantly higher than other report based on Lowenstein- Jensen [19]. The reason may be due to the skill proficiency of the operators as well as the different culture medium.
The progress in nucleic acid amplification technology has led to a breakthrough in early detection of pulmonary tuberculosis, which is remarkably superior to traditional sputum smear test. FDA has approved commercial nucleic acid diagnosis kits for tuberculosis laboratory diagnosis, and both BALF and sputum are recommended. BALF can increase the MTB nucleic acid positive rate of SSN-PTB [9]. 25 studies were reviewed, and the sensitivity/ specificity of tuberculosis laboratory diagnosis method by Xpert MTB/RIF and LAMP was 89%/98% and 93%/94% respectively [20]. The sensitivity of BALF for Xpert and smear microscopy was 80%-92.3% and the specificity was 95.8%-98.9% respectively [21- 23]. Among the SSN-PTB, the sensitivity and specificity of BALF tested by Xpert were 60% and 98%. BALF-Xpert could be used as a substitute for transbronchial lung biopsy in sputum-scarce and SSN-PTB [24]. BALF being used via Xpert MTB/RIF for rapid laboratory diagnosis in high TB burden countries can promote TB treatments [22,23]. LAMP is another nucleic acid amplification technology that has been used for rapid diagnosis of TB clinically. WHO recommends LAMP as an alternative method to microscope for the diagnosis of PTB in adults. In our study, the positive yield using BALF was 66.4% (168/253) and 61.0% (83/136) by method of Xpert MTB/RIF and LAMP respectively. Both were significant higher than Z-N-AFB-SM and culture. On the other hand, when comparing the BALF positive yields between the 2 groups patients of sputum-scarce and sufficient, there was no significance, which means BALF for APTB diagnosis are stable and repeatable.
The quality of BALF may be an important contributing factor for its diagnosis yield. BALF collected by well-trained operators had significant higher positivity. Probably attributed to this reason, there were unavoidable adverse events happened. However, it was reported that no significant adverse event was noted for patients or health-care staff recently [25].
It seems sputum-scarce and SSN-PTB or SCN-PTB is a common problem faced by clinicians. BALF has a similar sensitivity and specificity for APTB laboratory diagnosis. It can be used as a complementary diagnostic method for APTB with poor sputum. However, bronchoscopy is an invasive procedure associated with the risk of transmission of Tuberculosis (TB) and other infections. The proficiency of BALF collection is an important factor affecting the detection results.
We would like to thank all study participants who were involved and contributed to the data collection. We thank editors and reviewers for their warm works and valuable comments. We especially thank Prof. Shenjie Tang, Beijing Chest Hospital Affliated to Capital Medical University, Beijing, China. For his critical evaluation on the article
All data generated or analyzed during this study are included in this published article.
SY, QZ-S, LJ, JQ-G, YM-F and XF-Y designed the study. MH, SY, TX-L and WY-Y analyzed the data.MH, JQ-G, QQ and SY drafted the manuscript. SY, QZ-S, LL-W and LJ provided clinical data; TX-L and WY-Y operated the lab testing. SY supervised the study. All authors read and approved the final manuscript.
The study was approved by the Ethics Board of Chongqing Public Health Medical Center.
Citation: He Y, Yang S, Li T, Guo JQ, Fan YM, Han M, et al. (2022) Using Bronchoalveolar Lavage Fluid for Active Pulmonary Tuberculosis Laboratory Diagnosis. Mycobact Dis.12:295.
Received: 18-Aug-2022, Manuscript No. MDTL-22-18882; Editor assigned: 22-Aug-2022, Pre QC No. MDTL-22-18882 (PQ); Reviewed: 05-Sep-2022, QC No. MDTL-22-18882; Revised: 12-Sep-2022, Manuscript No. MDTL-22-18882 (R); Published: 21-Sep-2022 , DOI: 10.35248/2161-1068.22.12.295
Copyright: © 2022 He Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.