Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2013) Volume 4, Issue 2
Trichoderma species are well-appreciated filamentous fungi applied in agriculture for biological control and biofertilization purposes. One of the primary steps to screen for potent biological control isolates and/to study the effect of gene(s) on the biological control of phytopathogenic fungi and oomycetes is to perform confrontation or dual culture tests. Despite the comprehensiveness of the test, it still suffers from the lack of a reliable methodology for the mathematical data collection and statistical analyses. Here, different aspects of data collection are critically studied and new parameters are introduced. With this method the statistical comparison of different fungal biological control isolates and their counterpart phytopathogenic fungi as well as oomycetes becomes feasible. In the mean time, with this new approach, it becomes possible to statistically analyze the effect of different factors included in the medium of interaction. And with those interested in genetic studies on this type of interactions, the results of this study indicate the probable feasibility of statistical analysis of the possible impact of genetic manipulation and transformation of fungal biological control agents on the biological control of phytopathogenic fungi and oomycetes, and vice versa.
<Keywords: Confrontation test, Dual culture, Control, Trichoderma, Pythium
With the increase of human knowledge of and experience with the hazardous impacts of agricultural pesticides on the environment and human health, integrated pest management has changed to a choice of more attraction [1]. Biological control is a non-ignorable part of such a management system in modern agriculture [2]. Fungal biological control agents are among the most successful tools applied in the combat against plant pathogenic fungi [3-5] and Trichoderma-based fungicides are regarded as the most commonly used fungicides [6]. Some strains have the ability to reduce the severity of plant diseases by inhibiting plant pathogens, mainly in the soil or on plant roots, through their high antagonistic and mycoparasitic potential [7]. Effective Trichoderma isolates can play important roles in sustainable agriculture. These isolates would be able to (i) control phytopathogenic fungi [8], (ii) control plant nematodes [9-12], (iii) increase plant growth, development, and yield [13-22], (iv) induce plant systemic resistance of induced systemic resistance [22,23] acquired systemic resistance [24] against plant pests and diseases (v) increase plant resistance to abiological stresses [25,26], (vi) remediate polluted agricultural soils [7,27,28], (vii) improve soil environment [29-32], (viii) potentially control insect pests [33,34] and weeds [35-37].
Interestingly there are some data indicating the higher chance for biological control as Trichoderma isolates can be applied some while after given herbicides that are also of their effects against important oomycetous, ascomycetous, as well as basidiomycetous pathogens, although to this end field studies are required [38]. Such positive effects if also obtained under field conditions would be of peculiar practicality in integrated pest management systems as the dead bodies of the chemically controlled weeds could be inoculated and colonized by Trichoderma biological control isolates prior to the re-establishment of fungal pathogens and/re-start of the bioactivities of the herbicideimposed suppressed fungal pathogens. It is clear that Trichoderma fungi can effectively colonize dead plant materials thanks to their strong potential of the secretion of cellulases and other glucanases while some humidity is available during routine practice of soil preparation before cultivation. In the meantime and as implied in (vi), Trichoderma isolates as mycoremediants are able to remediate herbicide-treated soils-an effect of high importance if non-selective soil herbicides are applied. The colonized weed plant bodies would help Trichoderma isolates establishment and survival in soil [39,40]. Considering the beneficial roles Trichoderma isolates can play in eco-friendly agricultural systems, it would be very economic to search for the effective domestic isolates and use them in integrated pest management programs. After the isolation of numerous numbers of such isolates, their effectiveness in biological control programs has to be tested in the first screening step to reduce the number in a way biased for more antagonistic ones. Also with the attempts to generate genetic transformants more effective in biological control of phytopathogenic fungi (for scientific purposes or practical uses wherever it is legally allowed), or to understand the effect/role of the given genes on/in the process of biological control, the necessity of a quantitative method is increasingly felt. One of the conventionally applied screening tests is the confrontation test (also known as dual culture test) first introduced by Weindling [41]. The test is a comprehensive experiment that exhibits overall antagonistic potential of a fungal biological control agent, and can be applied after preliminary fast screening tests. The important constriction in the use of dual culture test lies in the collection of the experimental data. If pathogen growth inhibition is considered in the time of a pathogen full growth in check cultures, the full antagonistic potential of the fungal biological control agent will not probably be understood because full overgrowth of the biological control agent will often take a time period more than that required for the full growth of pathogen in check cultures. On the other hand, the growth inhibition percentage will be dependent on the plate diameter. If pathogen growth inhibition is to be considered in the time of confrontational contact between two opposed colonies [42], there will be a drawback with most of pathogens which their growth are temporarily promoted in the presence of fungal biological control agents. Another disadvantage of the confrontation test is the ignorance of time, so the speed of biological control activity is missed in pathogen growth inhibition percentage calculations. Vice versa, some researchers have only considered time period for full growth of a fungal biological control agent over a pathogen forgetting pathogen growth inhibition in the biological control. Some authors emphasize on the use of the temporal period that two colonies need in order to come in contact in dual culture test as well as pathogen growth inhibition percentage as applied in the conventional dual culture method. However, the time period required for coming in contact will not include the period of overgrowth on a pathogen colony. Here we attempt to develop a new methodology for data collection and analysis. Also, we will use the methodology for a comparative study on the biological control potentials of two T. asperelloides isolates against P. aphanidermatum at two different temperatures.
Fungal isolates
Two Trichoderma isolates T13 and T92 were isolated from the rhizosphere soil of tarragon (Artemisia dracunculus L.) in Hokmabad, Tabriz, Iran. These were selected out from more than 100 Trichoderma isolates based on the results obtained with some preliminary studies including different in vitro tests performed to evaluate their in vitro activities against various plant pathogens (fungi such as Fusarium spp., Macrophomina phaseolina, Rhizoctonia solani, and the oomycetous plant pathogen Pythium aphanidermatum), and to assess their abilities to grow on glycerol and mannitol, at different temperatures, and to grow on a layer of a long chain alkane. The specification of both isolates as T. asperelloides was performed at Vienna University of Technology, Vienna, Austria. An isolate of Pythium aphanidermatum was obtained from the collection of fungi available at the laboratory of Plant Pathology, Agricultural Faculty, Tarbiat Modares University, Tehran, Iran.
Confrontation tests to study the effect of temperature on the biological control
Malt extract agar plates in disposable plastic Petri dishes were used for dual inoculations with 5mm discs from 4 day old cultures grown at 25°C under dark conditions. The discs were put in a 5mm distance from the edge, opposed and on the same diagonal line. Three plates were considered for each fungus-fungus interaction so that the biological control potential of each Trichoderma isolate against Pythium aphanidermatum, an oomycetous soil born plant pathogen was studied. Two sets of each interaction were synchronously prepared and incubated in two incubators adjusted to 15°C and 25°C under dark conditions. Four parameters were determined per interaction: (i) days of the period after inoculation till two colonies came in contact (C); (ii) days of the period after inoculation till the fungal biological control agent fully grew over a pathogen colony (Z); (iii) days of the period after inoculation till the fungal biological control agent fully grew on the plate (M); and finally (iv) the radial distance (in mm) of pathogen colony growth between the edge of the inoculation disc and the marginal point of the colony located on the presumed diagonal line connecting centers of two discs in the plate (P). A parameter for pathogen resistance (R) to the biological control agent was defined based on the periods required for the full growth of a fungal biological control agent in the presence (Z) and absence (M) of pathogen. R was defined as the ratio (without any dimension) below:
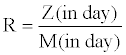
As the success of biological control decreases with the increase of pathogen resistance to the fungal biological control fungus and the increase of pathogen colony growth, therefore a new index was defined combining temporal parameters and pathogen growth parameter. Pakdaman’s biological control index (PBCI) was calculated following the equation below. The dimension of the index was determined as L-1 with the unit of mm-1.
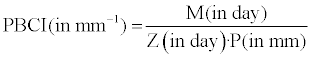
Statistical analysis of data
Data analysis was performed based on the experimental design of complete random blocks taking advantage of SAS 9.1.3 portable for Windows, SAS institute Inc. The means of parameters as well as indices were compared based on Tukey’s studentized range (HSD) tests (α=0.05). Also, the correlation among parameters was checked based on Pearson correlation coefficients using the above-mentioned software.
Analysis of data indicated that there were highly significant differences in the time period required for contact between pathogen and fungal biological control colonies (C) in the treatments (P<0.0001).
Comparison of mean C values through Tukey’s studentized range (HSD) test (α=0.05) indicated that both T. asperelloides isolates had statistically equal speed in growth toward P. aphanidermatum colonies, however, reduction of temperature from 25°C to 15°C caused the increase of C values from 2 to 4 days (Table 1).
| Treatment | Mean C value | Tukey grouping |
|---|---|---|
| 15°C Pa-T13 | 4 | A |
| 15°C Pa-T92 | 4 | A |
| 25°C Pa-T13 | 2 | B |
| 25°C Pa-T92 | 2 | B |
Table 1: Tukey’s studentized range test-based comparison (α=0.05) of average C values (in day) resulted from two different Trichoderma asperelloides isolates (T13 and T92) interactions with Pythium aphanidermatum (Pa) at two temperatures.
Similarly the differences in Z values of the treatments were also found very meaningful (P<0.0001). While two T. asperelloides isolates could grow over P. aphanidermatum colonies after an equal length of time period at 25°C (4 days), T92 could dominate the oomycetous pathogen sooner than T13 at 15°C (Table 2). Furthermore, while both biological control isolates had equal C values at 15°C, they had significantly different Z values under the same conditions.
| Treatment | Mean Z value | Tukey grouping |
|---|---|---|
| 15°C Pa-T13 | 12 | A |
| 15°C Pa-T92 | 11 | B |
| 25°C Pa-T13 | 4 | C |
| 25°C Pa-T92 | 4 | C |
Table 2: Tukey’s studentized range test-based comparison (α=0.05) of average Z values (in day) resulted from two different Trichoderma asperelloides isolates (T13 and T92) interactions with Pythium aphanidermatum (Pa) at two temperatures.
Other temporal parameter (M) was also found to be of very notably different values in different temperature-Trichoderma isolate combinations (P<0.0001). The effect of temperature on the fungal biological control agent full growth in check cultures was very meaningful (P<0.0001). Comparison of average M values showed both isolates of T. asperelloides were of equal M values at a given temperature and under equal conditions of the experiment but with the fall of temperature from 25°C down to 15°C, the average M value raised three times (Table 3).
| Treatment | Mean M value | Tukey grouping |
|---|---|---|
| 15°C Pa-T13 | 12 | A |
| 15°C Pa-T92 | 12 | A |
| 25°C Pa-T13 | 4 | B |
| 25°C Pa-T92 | 4 | B |
Table 3: Tukey’s studentized range test-based comparison (α=0.05) of average M values (in day) resulted from two different Trichoderma asperelloides isolates (T13 and T92) interactions with Pythium aphanidermatum (Pa) at two temperatures.
Notable differences were also found in the radial growth of P. aphanidermatum in the presence of T. asperelloides isolates T13 and T92 (P=0.0181), where two temperatures did not have any meaningful impact on pathogen growth in the interaction with biological control isolates which were of highly significant impact on the growth of P. aphanidermatum (P=0.0090). P. aphanidermatum grew more in the presence of biological control isolates at 25°C compared to 15°C (Table 4).
| Treatment | Mean P value | Tukey grouping |
|---|---|---|
| 25°C Pa-T13 | 47.6667 | A |
| 25°C Pa-T92 | 46.3333 | AB |
| 15°C Pa-T13 | 45.3333 | AB |
| 15°C Pa-T92 | 44.3333 | B |
Table 4: Tukey's studentized range test-based comparison (α=0.05) of average P values (in mm) resulted from two different Trichoderma asperelloides isolates (T13 and T92) interactions with Pythium aphanidermatum (Pa) at two temperatures.
With the parameter R, P. aphanidermatum exhibited highly significant differences in its resistance against T. asperelloides in different treatments (P<0.0001). The effect of temperature on the resistance was very meaningful (P<0.0001). The R values obtained in interactions to Trichoderma isolates were also highly different (P<0.0001). R values (calculated as the ratio of Z/M) equal to 1 indicate the equal facility of biological control isolate growth in the presence of the pathogenic fungus and in the absence of it on a rich substrate like malt extract agar. R values less than 1 indicate more facility of a biological control isolate in the presence of a pathogenic fungus compared to its growth on a medium and as a result of growth promoting effect of a pathogen. Based on the mean R values shown in Table 5, P. aphanidermatum could be regarded a so-easy-target for the T. asperelloides isolates, T13 and T92. P. aphanidermatum had a growth arising impact on T92 at 15°C.
| Treatment | Mean R value | Tukey grouping |
|---|---|---|
| 15°C Pa-T13 | 1.000 | A |
| 25°C Pa-T92 | 1.000 | A |
| 25°C Pa-T13 | 1.000 | A |
| 15°C Pa-T92 | 0.917 | B |
Table 5: Tukey’s studentized range test-based comparison (α=0.05) of average R values (without unit) resulted from two different Trichoderma asperelloides isolates (T13 and T92) interactions with Pythium aphanidermatum (Pa) at two temperatures.
Pakdaman’s biological control indices (PBCIs) were statistically highly different (P=0.0008), varying highly significantly as the result of temperature impact (P=0.0025) and T. asperelloides isolate effect (P=0.0123).
Comparison of average PBCI values indicated that T92 was more effective than T13 in the biological control of P. aphanidermatum, and the success of biological control was higher at 15°C (Table 6).
| Treatment | Mean PBCI value | Tukey grouping |
|---|---|---|
| 15°C Pa-T92 | 0.0235333 | A |
| 25°C Pa-T92 | 0.0223667 | B |
| 15°C Pa-T13 | 0.0220333 | BC |
| 25°C Pa-T13 | 0.0210000 | C |
Table 6: Tukey’s studentized range test-based comparison (α=0.05) of average PBCI values (in mm-1) resulted from two different Trichoderma asperelloides isolates (T13 and T92) interactions with Pythium aphanidermatum (Pa) at two temperatures.
Study of the possibility of correlations between C, Z, R, and P values in one hand and PBCI in other hand revealed no significant correlation between PBCI and each of the parameters respectively as C (R2=0.56561ns, P=0.0553), Z (R2=0.51192ns, P=0.0889), M (R2=0.56561ns, P=0.0553), and P (R2=0.0838ns, P=0.0838). However, a highly significant negative correlation was found between the parameter R and PBCI (R2=-0.77186**, P=0.0033). No correlation was found between P and other parameters including C (R2=-0.12309ns, P=0.7031), Z (R2=-0.14706ns, P=0.6483), M (R2=-0.12309ns, P=0.7031) and R (R2=-0.14213ns, P=0.6595).
The quantification of the confrontation or dual culture test in order to get access to analyzable data was an inescapable task in the comparison of fungal biological control isolates. Without any mathematical data in hand, precise analyses would be impossible. Here a new index for biological control was introduced based on the direct radial growth of the pathogen in the presence of a biological control agent (P), and the parameter for the resistance of a pathogen against a biological control agent applied. In as much as like what we observed with the interaction of two T. asperelloides isolates with P. aphanidermatum it was possible to have equal numbers for Z and M values, then resistance parameter (R) was defined as the ratio of Z/M. Pakdaman’s biological control index was defined as 1/P·R or M/P·Z. The index unit was determined as mm-1. Time has been considered inside the concept of R also a representative of phytopathogenic fungus resistance against biological control mechanism(s). Because of usually enfaced transitory promoting impact of the biological control agents on the growth of phytopathogenic fungi in one side, and the ignorance of full process of biological control and other problems in the calculation method conventionally applied in confrontation tests (explained in introduction), development of a new methodology for this important test was necessary. Considering the parameter C, this parameter seemed incapable in the revealing of the differences in the whole potentials of fungal biological control isolates. In contrast to C, another parameter Z comprehending whole of fungus-fungus interaction period reflected existent variation in the biological control potentials of isolates. C is only a part of the whole Z. Other point that is better to explain here is that the growth of pathogen at the time before C is not a suitable criterion. This is because of the promoting impact of Trichoderma isolates on the growth of plant pathogenic fungi as well oomycetes then followed with the invasion by Trichoderma isolates. Another point is that here in this method, no control was considered for the pathogen. The reason for this was that in most reactions pathogens were of very fast growth and could fill their own plates earlier than the end of the dual culture experiments and this can lead to error.
Using Pakdaman’s biological control index it was possible to get the mathematical data required for statistical analyses and Tukey’s studentized range (HSD) test was preferred to other tests because of its proper sensitivity in grouping of data means. The new methodology was found easy to perform and simple to understand. Using the new methodology it would be feasible to compare the performance of several biological control species and or isolates under various conditions and confronted with different phytopathogenic fungi.
Comparing the results from Tukey grouping based on C, Z, and P values with the results of PBCI, it is illustrated no of former three parameters can represent the level of biological control. Also, synchronous consideration of C and P did not end to the same result obtained based on PBCI. As C-based grouping could not show the differences between two biological control isolates, then Z was preferentially applied instead, and the formula for PBCI was developed using two temporal parameters to form a combined parameter, R representative of pathogen resistance under peculiar conditions. R values more than 1 indicate pathogen resistance to biological control mechanism(s) applied by a control agent in confrontation with a pathogen. R value equal to 1 indicates absence of any resistance by a pathogen. R values less than 1 indicate that a pathogen not only does not resist, but also promotes the growth and development of a biological control agent. With our study, P. aphanidermatum either did not show any resistance against T. asperelloides isolates, or it promoted the growth and development of biological control isolates. However, such a promoting impact was only observed with the isolate T92 and only at 15°C. This promotion might be because of the compensational provision of one or more growth promoting factors (such as vitamins, amino acids, etc) for a biological control isolate under special ecophysiological conditions. Additionally, such a promoting effect might be originally induced by peculiar biological control isolates under specific conditions.
Based on PBCI values obtained in this study, biological control of P. aphanidermatum with T92 isolate at 15°C would lead to more successful control. This finding is in agreement with thermophily of P. aphanidermatum and mesophily of T. asperelloides. Our findings indicate that PBCI can be regarded as a proper criterion useful in confrontation tests. Indeed, there is no need to change the formulas when other plant pathogens are studied and the introduced formulae have successfully been applied with other plant pathogens such as F. moniliforme, M. phaseolina, and R. solani.
The authors dedicate their intimate thanks to Mrs. Dr. Monika Komon- Zelazowska, Mrs. Dr. Irina S. Druzhinina and Mr. Prof. Dr. Christian Peter Kubicek from Research Area Gene Technology and Applied Biochemistry, Institute of Chemical Engineering, Vienna University of Technology, Vienna, Austria, who kindly helped through molecular identification of Trichoderma isolates applied in this research without any expectations.