Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Case Report - (2023)Volume 14, Issue 2
Background: We present a case of severe bilateral angle closure crisis in a patient using both topiramate and triptans with intraocular pressures that were refractory to initial medical therapy. We explore the mechanism of acute angle closure and additional factors that may be contributing to this specific presentation.
Case: In this case report, we present our clinical findings, review the mechanism of action of acute angle closure glaucoma, and explore well studied as well as theoretical treatments. We will review current literature citing topiramate induced acute angle closure glaucoma, sumatriptan acute angle closure glaucoma and investigate the possibility of a synergistic relationship between these medications as well as other sulfa based drugs.
Results: The literature suggests a well-known mechanism of angle closure induced by topiramate and sulfa drugs with the development of choroidal effusion causing anterior rotation of ciliary body, along with the lens-iris diaphragm, which leads to the closure of the angle. There is minimal literature on concomitant use of topiramate and triptans with an unknown synergistic relationship between these sulfa based medications. Additional literature review suggests a role of inflammation of the ciliary body in the closure of the angle. We treated our patient with an additional sulfabased drug acetazolamide and then steroids that improved her intraocular pressures and angle anatomy.
Conclusion: There is no known synergism between topiramate and triptan medications in acute angle closure, but the presentation, severity, and progression of acute angle closure with resistance to initial treatment in our patient may suggest a possible synergistic mechanism. The use of corticosteroids in topiramate or triptan induced acute angle closure may be indicated in the setting of the speculative role inflammation plays in angle closure.
Bilateral acute angle closure; Drug-induced; Glaucoma; Topiramate
Sulfa drugs are known to be associated with secondary acute angle closure glaucoma. Topiramate and Sumatriptan are both under the umbrella of sulfa drugs, but have distinct chemical structures, as Topiramate is a sulfate and Sumatriptan is a sulfonamide [1-7]. In the case we will present, the patient has concomitant use of both, which raises the question of a possible synergistic effect of multiple sulfa drugs and casts doubt on the use of acetazolamide, another non antibiotic sulfonamide drug in the treatment of an acute angle closure crisis.
There are likely two mechanisms for sulfa drug induced angle closure namely anatomical and inflammatory [8-11]. Topiramate specifically has many mechanisms of action including suppression of voltage-gated sodium channels, potentiation of potassium channels and Gamma-aminobutyric Acid (GABA), inhibition of voltage gated calcium channels and glutamate, dephosphorylation of membrane proteins such as receptors and channels and carbonic anhydrase inhibition. Sumatriptan, on the other hand, acts on the inhibition of potassium channels. Both are thought to cause activation of sympathetic pathways and inhibition of parasympathetic pathways, which ultimately leads to accumulation of fluid in the choroid and ciliary body due to increased permeability of ciliochoroidal vasculature. The fluid in the choroid and the edematous ciliary body move the lens-iris diaphragm anteriorly, and with the ciliary body rotating anteriorly, the angle becomes closed off [12,13]. Additionally, there is a decrease in zonular tension, which increases the thickness of the lens causing a myopic shift when there is forward movement and stiffening of the lens. Topiramate has also been cited to cause inflammation with associated cases of uveitis and hypopyon [14]. The bilateral angle closure and the apparent inflammation in the setting of topiramate and sumatriptan use are likely at play in the alarming initial presentation of our patient.
A 27-year-old Caucasian female presented to the emergency room with bilateral blurry vision and headache which started 2-3 days prior to presentation. Patient was prescribed both topiramate and sumatriptan within the past 1 month. In the 24 hours prior to presentation, she noted a progressive decrease in distance vision despite wearing her contact lenses. During this time, she maintained her near vision. She subsequently noticed faint halos when looking at bright lights and developed a significant brow ache. She took sumatriptan 50 mg twice a day beginning 2-3 days prior to presentation and continued to take topiramate 200 mg twice a day for migraine prophylaxis. The patient additionally had light sensitivity, but did not have any eye redness, flashes, floaters, or double vision. She was given and a dose of rizatriptan 5 mg for concern for ocular migraine at an outside emergency facility prior to transfer and reported worsening vision there. On arrival, she was found to have an intraocular pressure (IOP) in the right eye at 59 mmHg and left eye at 55 mmHg (Tonopen©). Her visual acuity on near card was 20/200 in both eyes. At this time patient was given latanoprost 0.005%, timolol 0.5%, and brimonidine 0.2% topically in both eyes. This was followed by Goldmann applanation with IOP of the right eye measuring 79 mmHg and left eye 74 mmHg.
On exam patient was also noted to have remarkably clear corneas. The anterior chamber of both eyes was found to be shallow. There were 1-2+ pigmented cell in each eye. The undilated fundus exam was remarkable for striated macula in both eyes. Patient was given 100 g of mannitol and about two hours later on Goldmann applanation, she was found to have a pressure of 74 mmHg in the right eye and 72 mmHg in the left eye. Due to the lack of response of the mannitol, patient was given Intravenous (IV) acetazolamide 250 mg, with a resulting pressure after 90 minutes with Goldmann applanation of 62 mmHg in the right and 64 mmHg in the left eye. There was no improvement in vision and the desired pressure drop was not achieved. Given the concern for a partial inflammatory mechanism of action resulting in angle closure and prior reports documenting improvement in IOP after corticosteroid use, patient was given IV methylprednisolone 500 mg, and the intraocular pressure on Goldmann applanation dropped dramatically to 48 mmHg in the right and 52 mmHg in the left within 20 minutes of the steroid dose [15].
Patient was then placed on topical eye drops, latanoprost 0.005%, combination drop of Dorzolamide HCl 2%/Timolol 0.5%, brimonidine 2% as well as prednisolone acetate 1%, and atropine 1% and was recommended to return to clinic the following morning. While the patient’s symptoms and pressure were being monitored closely, a B scan was conducted that showed choroidal effusion (Figure 1).
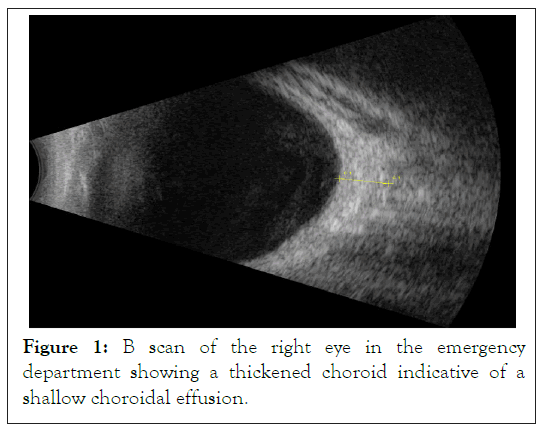
Figure 1: B scan of the right eye in the emergency department showing a thickened choroid indicative of a shallow choroidal effusion.
In clinic the following day, patient’s distance visual acuity continued to be poor at hand motion in both eyes, but pressures were stable at 22 mmHg in both eyes with Goldmann applanation. Patient was continued on the same therapy of eye drops. Patient returned four days after her original emergency department visit. During this visit distance visual acuity without correction improved to right eye 20/50 and left eye 20/40 (Snellen), and with new manifest refraction of spherical equivalent right eye -1.50 sphere and left eye -0.50 sphere, she was able to achieve a visual acuity of 20/20 of both eyes with stable intraocular pressures of 9 mmHg and 10 mmHg respectively. Slit lamp examination was notable for 4+ deep anterior chambers in both eyes as shown in Figures 2a and 2b with dilated exam again showing striae within the macula and newly noted tortuous vessels in both eyes with vascular congestion (Figures 2a and 2b).
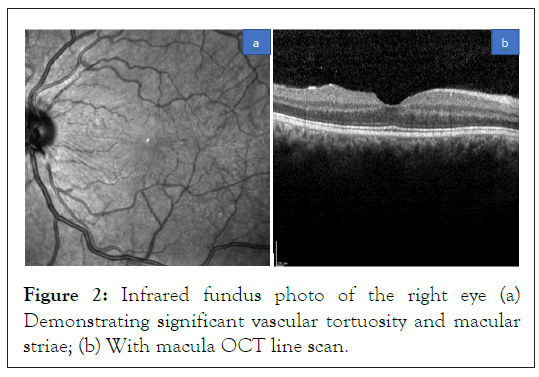
Figure 2: Infrared fundus photo of the right eye (a) Demonstrating significant vascular tortuosity and macular striae; (b) With macula OCT line scan.
With the notable tortuous vessels in both eyes, there was concern for an impending central retinal vein occlusion, but no hemorrhages or edema was identified. In addition to Optical Coherence Tomography (OCT) retina, an anterior segment OCT was conducted that demonstrated that the angle was now open. This coincided with the deep chamber seen on slit lamp examination (Figure 3).
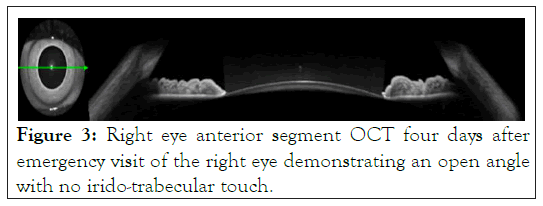
Figure 3: Right eye anterior segment OCT four days after emergency visit of the right eye demonstrating an open angle with no irido-trabecular touch.
Prior to this acute episode, patient utilized glasses and contact lenses with spherical equivalent right eye of -1.50 diopters and left eye of -1.25 diopters. Combination drop of Dorzolamide HCl 2%/Timolol 0.5% was stopped, while all others were continued. Twenty one days later, visual acuity without correction improved to right eye 20/30 and left eye 20/40, and to 20/20 with a -2 sphere in both eyes. She had stable IOP of 14 mmHg and 15 mmHg of the right eye and left eye respectively. There were no changes on slit lamp or dilated exam in either eye from prior clinic visit. Patient was stopped on all drops. At two week follow up, patient had visual acuity without correction stable 20/30 and left eye 20/40, and with new manifest refraction of spherical equivalent right eye -2.50 diopters and left eye -2.00 diopters with a visual acuity of 20/20 of both eyes with stable IOPof 13 mmHg of both eyes respectively.
Patients, most typically young women, are being prescribed topiramate and triptans for migraine prophylaxis and migraine abortive therapy respectively. Both topiramate and triptans have been identified as medications that can induce angle closure, but a synergistic relationship is not known. This patient appeared to have worsening of symptoms with additional dose of rizatriptan and additionally had an almost refractory decrease in IOP, notably with minimal decrease in initial pressure with mannitol. It is plausible that the dual therapy had a remarkably severe and sight threatening presentation with optic neuropathy and impending compressive vascular occlusion.
These medications, although acting on different ion channels, are thought to both cause ciliary body rotation with the iris lens diaphragm moving anteriorly and an associated myopic shift to cause angle closure. In addition, there appears to be evidence to suggest inflammation of the ciliary body is playing a role in the angle closure. There has been a documented case of topiramate induced angle closure with formation of hypopyon as well as other literature demonstrating decrease in IOP with use of methylprednisolone in topiramate induced angle closure glaucoma. The large decrease in IOP seen in our patient after methylprednisolone only further suggests a component of inflammation, which when suppressed, resulted in a lower IOP in each eye.
Although there was significant concern with a possible sensitivity to sulfa drugs in our case, we utilized acetazolamide (after an initial minimal response to mannitol), which is additionally a sulfa based drug. Our patient did not have an additional synergistic effect, but demonstrated a decrease in IOP of about 10 mmHg in both eyes after about 90 minutes. As the patient did not have an apparent negative reaction to the acetazolamide, patient was sent home on latanoprost 0.005%, brimonidine 0.2%, and combination of timolol 0.5% and dorzolamide 2%, despite the fact that the latter medication is sulfa based. We question whether sulfa drugs should be an option for our patient in the future for her headache and migraine treatment. An additional question remains whether patient demonstrated residual myopia from the episode as has been reported or simply needed an updated refraction at this time [16]. Ultimately the patient had good visual recovery with close follow up and swift treatment of the elevated IOP.
The use of topiramate and triptans for migraine treatment are extremely common, but the risks of angle closure should be discussed when prescribing both medications, particularly when prescribing them together. Emergency Department physicians may want to consider an angle closure etiology in patients on one of these medications and avoid other potentially synergistic medications. Although treatment typically consists of eliminating the offending agent, glaucoma medications, and possible mannitol, this case and other literature reports the use of corticosteroids in the management of topiramate or triptan induced acute angle closure glaucoma.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Gulhar S, Mays C, Bansal S (2023) Topiramate and Triptan Concomitant use in Acute Angle Closure Glaucoma. J Clin Exp Ophthalmol. 14:944.
Received: 27-Feb-2023, Manuscript No. JCEO-23-21700; Editor assigned: 01-Mar-2023, Pre QC No. JCEO-23-21700 (PQ); Reviewed: 15-Mar-2023, QC No. JCEO-23-21700; Revised: 22-Mar-2023, Manuscript No. JCEO-23-21700 (R); Published: 30-Mar-2023 , DOI: 10.35248/2155-9570.23.14.944
Copyright: © 2023 Gulhar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.