Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2013) Volume 4, Issue 1
Densities and Ultrasonic velocities of the binary liquid mixtures of Anisicaldehyde with Methyl Acetate (MA) Ethyl Acetate (EA) and Butyl Acetate (BA) have been measured at a temperature range from 303.15 to 318.15 K with an interval of 5 K, over the entire composition of mole fractions. The theoretical values of ultrasonic velocity were evaluated using the Nomoto’s relation (UNR), Impedance relation (UIR), Ideal mixing relation (UIMR), Rao’s specific velocity relation (UR) Junjie’s relation (UJR) and Danusso model (UD). The molecular interaction parameter (χ), Chi-square value, average percentage error (APE) have been evaluated to find the best applicable theory. The variation of this interaction parameter with the mole fraction of common compound has been discussed in terms of molecular interactions.
Keywords: Ultrasonic velocity; Anisic aldehyde; Acetates; Chisquare test; Molecular interaction parameter
Theoretical evaluation of ultrasonic velocity in binary liquid mixtures and its correlation to study molecular interaction has been successfully done in recent years. Ultrasonic velocities of liquid mixtures containing polar and non-polar groups are of considerable importance in understanding intermolecular interaction between component molecules, and they find applications in several industrial and technological processes. Ultrasonic velocities in liquid mixtures have been calculated and compared with experimental values using various theories [1-9]. Comparison of evaluated theoretical velocities with those obtained experimentally is expected to reveal the nature of interaction between component molecules in the mixtures. Such theoretical study is useful in defining a comprehensive theoretical model for a specific liquid mixture. They are also valuable in testing various theories of liquid state. Many researchers compared the experimental values of ultrasonic velocities with theoretically evaluated values for organic liquid mixtures using different theories/ models [10-19] like Nomoto [1], Van Dael and Vangael [2], impedance relation [7], Rao’s specific velocity [8] and Junjie [5].
The aim of the present investigation is to compare the experimentally determined ultrasonic sound velocity in binary liquid mixtures with those of theoretical relations/models like Nomoto, Van Dael and Vangael, impedance relation, Rao’s specific velocity, Junjie and Danusso model at different temperatures. In the present investigation, Anisic aldehyde is mixed with esters like methyl acetate, ethyl acetate and n- butyl acetate at different mole fractions, to study the extent of interactions between dissimilar molecules. The relative applicability of ultrasonic theories to the present systems has been checked and discussed. The results are explained and discussed in terms of intermolecular interactions occurring in these binary systems. The deviation in the variation of U2exp/ U2imx, average percentage error, (APE), Chi-square test for goodness of fit, from unity have also been evaluated to further explain the non-ideality of the system.
Anisic aldehyde, methyl acetate, ethyl acetate and n- butyl acetate from Merk were purified as described in the literature [17,18]. The density was measured with apycnometer having a bulb volume of about 25 cm3 and an internal capillary diameter of about 1 mm. The density was then determined from the mass of the sample and the volume of pycnometer. Uncertainties in density determinations were estimated to be within ± 0.0001 g cm-3. The ultrasonic velocity of sound (U) is measured using an ultrasonic interferometer (Mittal Enterprises, New Delhi model F05) operating at 2 MHz. The measured speeds of sound have a precision of 0.8 m.sec-1 and an uncertainty less than ± 0.1 m.sec-1. Thetemperature stability was maintained within ± 0.01K by circulating water bath around the measuring cell through a pump.
Nomoto Equation
As per the relation proposed by Rao [19], the ratio of temperature coefficients of sound velocity U and molar volume V remains almost constant for pure liquids:
[(1/U) (dU/dT)] / [(1/V) (dV/dT)] = -3 (1)
where T is the absolute temperature. Integrating the above equation leads to
VU1/3 = const = M/ρU1/3 = R (2)
where M is molecular weight and ρ is density. The constant R is called the molar sound velocity or Rao’s constant. It was found to be an additive relation that is it can be calculated as a sum of increments from the atoms/atom groups in the molecule and from the chemical bonds under consideration. On assuming the additivity of molar sound velocity (R) and no volume change on mixing, Nomoto established the following relation for a liquid mixture
R = M/ρU1/3 (3)
where U and ρ are determined experimentally and M is the mean molecular weight in abinary liquid mixture
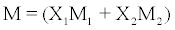 (4)
(4)
where M1 and M2 are molecular weights of constituent components. Simple manipulation yields the following relation
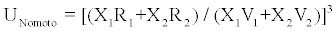 (5)
(5)
The Van Dael and Vangael Equation
The ideal mixing theory advanced by Van Dael and Vangael suggested the following equation
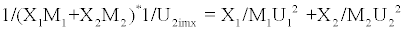 (6)
(6)
Where Uimx is the ideal mixing ultrasonic velocity in liquid mixture. U1 and U2 are ultrasonic velocities in species [20].
The Impedance relation
The Impedance relation is based on the forces of resistance for sound velocity by the interacting molecules and is given as
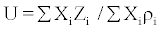 (7)
(7)
where Xi mole fraction, ri, is the density of the mixture and Zi is the acoustic impedance.
The Rao’s specific velocity method relation
Rao’s specific velocity model is given as
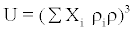 (8)
(8)
where Xi mole fraction, Ui is the ultrasonic velocity, ri is the density of the mixture, ri is the Rao’s specific sound velocity = Ui1/3/ri and Zi is the acoustic impedance.
The Jungie equation
Jungie has proposed an equation for the evaluation of velocity of sound in a given liquid mixtures, and is represented by
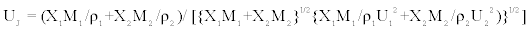 (9)
(9)
where M1, M2 are molecular weights of constituent components. r1 and r2 are the densities of constituent components.
Danusso model
Danusso model of velocity of ultrasonic waves is given by
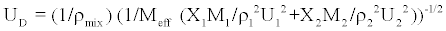 (10)
(10)
The validity of the above models/theories in evaluating the ultrasonic velocity is verified by various tests/error determinations, which would help us in finding the appropriate model or theory applicable the mixture under study. Following are the adopted tests/ errors determined for the binary under study [21].
Chi-square test for goodness of fit
According to Karl Pearson Chi-square value is evaluated for the binary liquid mixtures under study using the formula
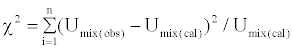 (11)
(11)
where n is the number of data used.
Average percentage error (APE)
The Average percentage error is calculated using the relation
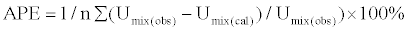 (12)
(12)
where n is the number of data used.
Umix (obs) = experimental values of ultrasonic velocities
Umix (cal) = computed values of ultrasonic velocities
Molecular associations
The degree of intermolecular interaction or molecular association is given by
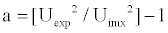 (13)
(13)
Anisic Aldehyde, also known as para-methoxybenzaldehyde is slightly polar (CH=O group). Oxygen is more electronegative than carbon so it has a tendency to pull electrons in a carbon-oxygen bond towards itself. Acetates are also polar compounds with high dipole moments. Hence while mixing the component molecules of binary mixture are expected to have dipole - dipole associations and they associate with AA, in the order MA>EA>BA. As the carbon chain length increases from MA to BA the strength of interaction between the mixing molecules decreases, due to the steric hindrance of long carbon chain length. The experimental values of ultrasonic velocity for the system along with theoretical values and percentage deviations for Nomoto’s Relation (UN), Vandeal Vangael Ideal Mixing Relation (UImix), Impedance Dependence Relation (UI), Rao’s specific velocity method (URao) Junjie’s relation (UJ) and Danusso (UD) are compared for all the three binaries. Table 1, denotes the evaluated velocity values from different theories adopted under study along with experimental values. Data reveals that the velocities computed from Nomoto relation (UN) exhibit more satisfactory agreement with the experimental valuesin the temperature range 303.15 K-318.15 K than other approaches in the binary systems. The agreement between theoretical velocities of Nomoto’s relation in all the three binary systems suggests that R is additive property in all the three systems. It is observed that the experimental values show deviation with the theoretical values of ultrasonic velocities which confirms the existence of molecular interactions. Tables 1-3 show the values of ultrasonic velocity computed by various theories along with experimental values (U). There are variations between the evaluated and experimental values. This may be due to interactions occurring between the hetero molecules of the binaries as mentioned above. From the observed values of all three systems, there is a good agreement between theoretical and experimental values through Nomoto’s relation followed by Impedance Relation. There are higher variations in some intermediate concentration range suggesting the existence of strong tendency of association between component molecules as a result of hydrogen bonding [22]. Nomoto’s theory proposes that the volume does not change upon mixing. Therefore, no interaction between the components of liquid mixtures has been taken into account. Similarly, the assumption for the formation of ideal mixing relation is that, the ratios of specific heats of ideal mixtures and the volumes are also equal. Again no molecular interactions are taken into account. But upon mixing, interactions between the molecules occur because of the presence of various types of forces such as dispersion forces, charge transfer, hydrogen bonding dipole-dipole and dipole-induced dipole interactions. Thus, the observed deviation of theoretical values of velocity from the experimental values shows that the molecular interactions are taking place between the unlike molecules [23]. The deviation of the ratio U2/Uimx2 from unity and its variation as a function of mole fraction of AA is a direct measure of non-ideality of the system as a consequence of association or other type of interactions. Tables 4-6 presents the calculated percentage deviations and interaction parameters and variation of U2/Uimx2 with the mole fraction of Anisic Aldehyde with three alkyl acetates. From the Tables it is observed that maximum positive deviation at 0.5 mole fraction of AA for AA + MA, EA systems for U2/Uimx2 is observed and AA + BA system showed at 0.6 mole fraction of AA at all the temperatures. The ratio U2/Uimx2 is an important tool to measure the non-ideality in the mixtures especially in such cases where the properties other than sound velocity are not known [24]. Figure 1 represent the variation of U2/Uimx2 with the mole fraction of anisic aldehyde for all three binary systems studied, and the ratio of U2/Uimx2 gives an idea of extent of interaction taking place between molecules of the mixtures. It is positive for three systems and infers strong interactions between the components. The percentage of deviation in velocity is reflecting both negative and positive magnitudes, indicating non ideal behavior of liquid mixtures. The evaluated interaction parameters are positive for all the systems, indicating stronger interactions between the mixing molecules, which increase from BA to MA. This suggests somewhat stronger interaction of Anisic Aldehyde with MA in comparison to other acetates [25]. The negative values indicate the dominance of dispersion forces arising from the breakage of hydrogen bonds in the associates [26]. A positive value of a in all the system clearly indicates the existence of strong tendency for the formation of association in mixture through dipole-dipole interactions higher values of percentage deviation indicates maximum departure of the particular theory from experiment at that particular concentration and magnitude of the chi-square value finally determines the overall validity of the theory [27]. The chi square values along with average percentage error are given in table 7. On the whole, all the theoretical models fairly predicted ultrasonic velocities, are reasonably close to the experimental values for and the three binary mixtures reported inthis work, thus showing the validity of studied theoretical models for binary mixtures. The predictive abilities of various ultrasonic theories discussed above, depend upon the strength interaction prevailing in a system; these theories generally fail to predict accurately the ultrasonic velocities where strong interactions are supposed to exist and the average absolute percentage relative deviation is small in systems where the interactions are less or nil [25].
| Compound | Experimental. ρx10-3 kg/m3 | Literature ρx10-3 kg/m3 | Experimental u/ m.s-1 | Literature u/ m.s-1 |
|---|---|---|---|---|
| A.A M.A E.A B.A |
1.1204 0.9207 0.8884 0.8695 |
1.1200a 0.9209b 0.8884d 0.8712d |
1543.5 1106.5 1122.0 1176.1 |
1542.0a 1118.0c 1123.0c 1176d |
Table 1: Comparison of density ρ, and ultrasonic velocity U, of the pure liquids with literature data at 303.15 K.
| X1 | Uexp ms-1 |
UNR ms-1 |
Uimx ms-1 |
UIR ms-1 |
UR ms-1 |
UJ ms-1 |
UD ms-1 |
|---|---|---|---|---|---|---|---|
| 303.15 K | |||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
1106.50 1157.00 1200.00 1240.50 1281.00 1322.70 1361.55 1403.60 1445.40 1496.08 1543.50 |
1106.50 1145.91 1186.25 1227.52 1269.73 1312.91 1357.05 1402.17 1448.27 1495.38 1543.50 |
1106.50 1103.62 1104.01 1108.45 1118.09 1134.63 1160.77 1200.95 1263.24 1364.04 1543.50 |
1106.50 1142.40 1179.76 1218.67 1259.21 1301.49 1345.64 1391.77 1440.02 1490.54 1543.50 |
1106.49 1171.42 1230.50 1285.70 1338.14 1387.54 1432.48 1471.24 1502.91 1527.83 1543.48 |
1106.50 1127.75 1151.84 1179.21 1210.44 1246.27 1287.66 1335.90 1392.76 1460.74 1543.50 |
1106.50 1124.18 1146.51 1173.03 1203.75 1239.28 1280.78 1329.81 1388.18 1458.02 1543.50 |
| 308.15 K | |||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
1099.60 1152.50 1195.90 1235.24 1274.00 1313.50 1352.06 1390.55 1429.00 1475.20 1516.93 |
1099.60 1137.41 1176.07 1215.58 1255.96 1297.22 1339.36 1382.39 1426.32 1471.17 1516.93 |
1099.60 1096.49 1096.58 1100.63 1109.75 1125.58 1150.72 1189.44 1249.41 1346.12 1516.93 |
1099.60 1133.85 1169.50 1206.63 1245.32 1285.70 1327.85 1371.92 1418.02 1466.30 1516.93 |
1099.59 1164.38 1224.87 1280.77 1331.94 1378.37 1419.94 1455.97 1484.99 1505.11 1516.95 |
1099.60 1120.22 1143.56 1170.04 1200.18 1234.67 1274.39 1320.53 1374.67 1439.06 1516.93 |
1099.60 1116.29 1137.02 1161.99 1191.52 1226.06 1266.31 1313.40 1369.13 1436.09 1516.93 |
| 313.15 K | |||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
1090.50 1149.70 1193.25 1231.50 1271.39 1312.40 1349.80 1388.25 1426.80 1470.00 1509.29 |
1090.50 1128.35 1167.06 1206.66 1247.14 1288.53 1330.82 1374.03 1418.17 1463.26 1509.29 |
1090.50 1087.49 1087.67 1091.79 1100.96 1116.85 1142.02 1180.77 1240.81 1337.72 1509.29 |
1090.50 1124.92 1160.74 1198.03 1236.89 1277.42 1319.72 1363.92 1410.15 1458.56 1509.29 |
1090.49 1155.79 1216.82 1273.90 1326.22 1374.18 1416.95 1452.68 1481.61 1501.08 1509.29 |
1090.50 1111.02 1134.27 1160.67 1190.76 1225.25 1265.03 1311.31 1365.74 1430.61 1509.29 |
1090.50 1106.92 1127.40 1151.97 1181.15 1215.27 1255.26 1302.61 1358.66 1426.31 1509.29 |
| 318.15 K | |||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
1084.60 1147.00 1190.20 1228.30 1266.90 1306.50 1342.00 1379.63 1417.50 1459.03 1492.06 |
1084.60 1121.30 1158.87 1197.31 1236.65 1276.89 1318.04 1360.12 1403.14 1447.12 1492.06 |
1084.60 1081.47 1081.48 1085.39 1094.26 1109.73 1134.31 1172.19 1230.86 1325.38 1492.00 |
1084.60 1118.27 1153.27 1189.67 1227.56 1267.04 1308.20 1351.16 1396.03 1442.95 1492.06 |
1084.60 1150.56 1213.29 1270.45 1322.93 1370.27 1411.99 1446.71 1473.40 1489.47 1492.05 |
1084.60 1104.36 1126.80 1152.35 1181.52 1215.02 1253.71 1298.78 1351.85 1415.18 1492.06 |
1084.60 1100.05 1119.19 1142.82 1170.92 1204.02 1242.92 1288.93 1343.71 1410.06 1492.06 |
Table 2: Experimental velocities (U/m.sec-1), theoretical velocities ((Ux/m.sec-1) for the system anisic aldehyde (AA) +methyl acetate (MA).
| X1 | Uexp ms-1 |
UNR ms-1 |
Uimx ms-1 |
UIR ms-1 |
UR ms-1 |
UJ ms-1 |
UD ms-1 |
|---|---|---|---|---|---|---|---|
| 303.15 K | |||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
1122.00 1170.20 1215.10 1259.70 1303.00 1342.50 1381.63 1421.70 1462.25 1503.53 1543.50 |
1122.00 1160.19 1199.23 1239.13 1279.92 1321.58 1364.14 1407.60 1451.98 1497.27 1543.50 |
1122.00 1128.71 1138.88 1153.17 1172.52 1198.21 1232.15 1277.17 1337.82 1421.79 1543.50 |
1121.99 1165.26 1208.27 1251.03 1293.54 1335.80 1377.82 1419.60 1461.14 1502.44 1543.50 |
1122.00 1184.78 1243.27 1298.34 1350.00 1397.56 1440.02 1476.38 1506.07 1528.77 1543.48 |
1122.00 1141.28 1163.49 1189.07 1218.58 1252.77 1292.61 1339.38 1394.90 1461.69 1543.50 |
1122.00 1138.73 1159.40 1183.94 1212.72 1246.49 1286.36 1333.73 1390.46 1459.05 1543.50 |
| 308.15 K | |||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
1101.50 1151.50 1197.20 1241.80 1284.48 1323.50 1361.50 1401.20 1440.30 1479.70 1516.93 |
1101.50 1139.10 1177.54 1216.85 1257.03 1298.09 1340.03 1382.88 1426.64 1471.32 1516.93 |
1101.50 1108.13 1118.15 1132.23 1151.29 1176.59 1210.02 1254.37 1314.12 1396.89 1516.93 |
1101.50 1144.17 1186.58 1228.74 1270.65 1312.31 1353.72 1394.88 1435.81 1476.49 1516.93 |
1101.50 1163.32 1221.66 1276.58 1327.66 1374.20 1415.43 1450.80 1480.00 1502.61 1516.92 |
1101.50 1120.43 1142.25 1167.39 1196.42 1230.07 1269.29 1315.39 1370.14 1436.07 1516.93 |
1101.50 1117.92 1138.00 1161.92 1190.18 1223.52 1262.96 1309.79 1365.76 1433.40 1516.93 |
| 313.15 K | |||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
1082.50 1136.50 1184.00 1230.10 1273.20 1314.20 1353.90 1393.77 1434.00 1473.80 1509.29 |
1080.65 1119.21 1158.69 1199.11 1240.48 1282.81 1326.11 1370.40 1415.69 1461.98 1509.29 |
1082.42 1089.33 1099.66 1114.08 1133.53 1159.32 1193.38 1238.64 1299.79 1384.89 1509.29 |
1082.50 1126.38 1169.98 1213.32 1256.38 1299.18 1341.72 1384.00 1426.02 1467.78 1509.29 |
1082.49 1144.94 1203.53 1259.00 1311.16 1359.10 1401.71 1438.24 1468.56 1492.77 1509.29 |
1082.50 1101.64 1123.72 1149.20 1178.69 1212.95 1253.01 1300.26 1356.64 1424.93 1509.29 |
1082.50 1098.99 1119.38 1143.66 1172.29 1206.12 1246.35 1294.42 1352.18 1422.27 1509.29 |
| 318.15 K | |||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
1055.90 1112.50 1160.00 1205.30 1249.50 1293.00 1334.50 1376.00 1417.50 1458.00 1492.06 |
1056.09 1095.03 1134.96 1175.91 1217.87 1260.88 1304.94 1350.08 1396.30 1443.62 1492.06 |
1055.94 1063.10 1073.66 1088.32 1108.03 1134.14 1168.63 1214.54 1276.74 1363.76 1492.06 |
1055.90 1100.93 1145.63 1190.01 1234.08 1277.84 1321.28 1364.43 1407.27 1449.81 1492.06 |
1055.89 1118.93 1180.12 1233.82 1287.22 1335.15 1378.88 1417.68 1450.56 1474.31 1492.05 |
1055.90 1074.90 1096.90 1122.39 1151.99 1186.53 1227.13 1275.29 1333.15 1403.82 1492.06 |
1055.90 1072.12 1091.73 1116.72 1145.31 1179.69 1220.43 1269.09 1327.95 1401.01 1492.06 |
Table 3: Experimental velocities (U/m.sec-1), theoretical velocities ((Ux/m.sec-1) for the system anisaldehyde+ ethyl acetate (EA).
| X1 | Uexp ms-1 |
UNR ms-1 |
Uimx ms-1 |
UIR ms-1 |
UR ms-1 |
UJ ms-1 |
UD ms-1 |
|---|---|---|---|---|---|---|---|
| 303.15 K | |||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
1176.10 1227.35 1274.50 1319.00 1359.90 1397.50 1430.00 1461.48 1490.80 1519.20 1543.50 |
1176.10 1209.81 1244.18 1279.21 1314.90 1351.27 1388.33 1426.07 1464.51 1503.65 1543.50 |
1176.10 1198.59 1223.28 1250.38 1280.21 1313.12 1349.53 1390.00 1435.20 1486.00 1543.50 |
1176.10 1225.98 1272.04 1314.72 1354.36 1391.29 1425.77 1458.04 1488.30 1516.74 1543.50 |
1176.10 1224.22 1270.41 1314.39 1356.05 1395.17 1431.38 1464.23 1493.51 1519.54 1543.50 |
1176.10 1192.68 1212.02 1234.48 1260.54 1290.79 1326.01 1367.23 1415.81 1473.67 1543.50 |
1176.10 1190.95 1208.87 1230.24 1255.53 1285.35 1320.57 1362.33 1412.09 1471.69 1543.50 |
| 308.15 K | |||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
1154.80 1207.30 1255.63 1300.17 1340.02 1377.40 1408.72 1439.10 1467.65 1493.74 1516.93 |
1154.80 1188.06 1221.96 1256.50 1291.70 1327.55 1364.07 1401.26 1439.13 1477.69 1516.93 |
1154.80 1176.95 1201.26 1227.96 1257.35 1289.77 1325.66 1365.55 1410.11 1460.20 1516.93 |
1154.80 1203.93 1249.31 1291.37 1330.44 1366.84 1400.83 1432.64 1462.48 1490.53 1516.93 |
1154.79 1203.27 1248.64 1291.74 1333.26 1371.79 1407.50 1439.62 1467.60 1493.65 1516.93 |
1154.80 1171.20 1190.31 1212.50 1238.22 1268.06 1302.79 1343.40 1391.25 1448.21 1516.93 |
1154.80 1169.12 1186.87 1208.04 1232.84 1262.26 1296.98 1338.22 1387.52 1446.10 1516.93 |
| 313.15 K | |||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
1137.00 1192.90 1242.20 1287.80 1328.88 1367.47 1399.60 1430.10 1460.00 1486.55 1509.29 |
1137.00 1171.11 1205.90 1241.38 1277.53 1314.39 1351.94 1390.20 1429.17 1468.87 1509.29 |
1137.00 1159.50 1184.21 1211.41 1241.40 1274.55 1311.34 1352.36 1398.34 1450.24 1509.29 |
1137.00 1187.44 1234.06 1277.27 1317.43 1354.85 1389.81 1422.54 1453.24 1482.11 1509.29 |
1137.01 1186.49 1232.23 1276.27 1318.30 1358.22 1395.34 1428.75 1458.32 1486.02 1509.29 |
1137.00 1153.80 1173.35 1196.03 1222.33 1252.86 1288.42 1330.09 1379.31 1438.08 1509.29 |
1137.00 1151.55 1169.81 1191.42 1216.91 1246.89 1282.34 1324.61 1375.18 1435.48 1509.29 |
| 318.15 K | |||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
1106.20 1166.00 1216.70 1263.83 1305.90 1345.69 1380.11 1412.63 1442.32 1469.25 1492.06 |
1106.20 1141.34 1177.23 1213.86 1251.26 1289.43 1328.37 1368.10 1408.61 1449.93 1492.06 |
1106.20 1129.08 1154.27 1182.05 1212.77 1246.85 1284.82 1327.34 1375.25 1429.68 1492.06 |
1106.20 1158.54 1206.89 1251.69 1293.32 1332.11 1368.33 1402.23 1434.03 1463.92 1492.06 |
1106.20 1156.29 1203.36 1248.35 1291.36 1332.64 1370.94 1406.06 1438.24 1467.18 1492.05 |
1106.20 1123.30 1143.21 1166.35 1193.24 1224.55 1261.14 1304.21 1355.36 1416.87 1492.06 |
1106.20 1121.05 1139.51 1161.58 1187.71 1218.45 1255.02 1298.64 1350.88 1414.12 1492.06 |
Table 4: Experimental velocities (U/m.sec-
| X1 | %UNR | %Uimx | %UIR | %UR | %UJ | %UD | U2/U2imx | α |
|---|---|---|---|---|---|---|---|---|
| 303.15 K | ||||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
0.0000 -0.9585 -1.1462 -1.0467 -0.8796 -0.7405 -0.3308 -0.1022 0.1989 -0.0466 0.0000 |
0.0000 -4.6133 -7.9990 -10.6448 -12.7175 -14.2184 -14.7466 -14.4378 -12.6024 -8.8256 0.0000 |
-0.0004 -1.2615 -1.6863 -1.7601 -1.7013 -1.6034 -1.1688 -0.8431 -0.3723 -0.3702 0.0002 |
-0.0012 1.2464 2.5416 3.6440 4.4605 4.9017 5.2096 4.8191 3.9788 2.1221 -0.0011 |
0.0000 -2.5280 -4.0137 -4.9410 -5.5084 -5.7786 -5.4272 -4.8236 -3.6421 -2.3625 0.0000 |
0.0000 -2.8369 -4.4573 -5.4386 -6.0301 -6.3065 -5.9321 -5.2568 -3.9586 -2.5443 0.0000 |
1.0000 1.0991 1.1814 1.2524 1.3126 1.3590 1.3759 1.3660 1.3092 1.2030 1.0000 |
0.0000 0.0991 0.1814 0.2524 0.3126 0.3590 0.3759 0.3660 0.3092 0.2030 0.0000 |
| 308.15 K | ||||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
0.0002 -1.3091 -1.6582 -1.5913 -1.4157 -1.2395 -0.9394 -0.5868 -0.1873 -0.2733 0.0001 |
0.0000 -4.8595 -8.3049 -10.8974 -12.8926 -14.3065 -14.8911 -14.4627 -12.5675 -8.7500 0.0000 |
-0.0003 -1.6180 -2.2076 -2.3165 -2.2509 -2.1168 -1.7904 -1.3401 -0.7685 -0.6031 0.0000 |
-0.0005 1.0305 2.4222 3.6863 4.5480 4.9386 5.0202 4.7046 3.9183 2.0275 0.0014 |
0.0000 -2.8005 -4.3764 -5.2787 -5.7946 -6.0017 -5.7444 -5.0355 -3.8018 -2.4498 0.0000 |
0.0000 -3.1417 -4.9237 -5.9304 -6.4742 -6.6570 -6.3425 -5.5483 -4.1893 -2.6511 0.0090 |
1.0000 1.1048 1.1893 1.2596 1.3179 1.3618 1.3805 1.3668 1.3081 1.2010 1.0000 |
0.0000 0.1048 0.1893 0.2596 0.3179 0.3618 0.3805 0.3668 0.3081 0.2010 0.0000 |
| 313.15 K | ||||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
0.0000 -1.8571 -2.1946 -2.0172 -1.9072 -1.8191 -1.4062 -1.0242 -0.6045 -0.4587 0.0000 |
0.0000 -5.4107 -8.8484 -11.3448 -13.4046 -14.9004 -15.3933 -14.9453 -13.0355 -8.9985 0.0000 |
-0.0005 -2.1551 -2.7246 -2.7178 -2.7137 -2.6657 -2.2285 -1.7523 -1.1666 -0.7784 -0.0001 |
-0.0008 0.5293 1.9751 3.4428 4.3128 4.7075 4.9746 4.6411 3.8412 2.1140 0.0000 |
0.0000 -3.3643 -4.9431 -5.7517 -6.3415 -6.6404 -6.2800 -5.5419 -4.2797 -2.6797 0.0000 |
0.0000 -3.7208 -5.5184 -6.4579 -7.0978 -7.4006 -7.0042 -6.1691 -4.7755 -2.9724 0.0000 |
1.0000 1.1177 1.2036 1.2723 1.3336 1.3808 1.3970 1.3823 1.3223 1.2075 1.0000 |
0.0000 0.1177 0.2036 0.2723 0.3336 0.3808 0.3970 0.3823 0.3223 0.2075 0.0000 |
| 318.15 K | ||||||||
| 0.0000 0.0685 0.1420 0.2210 0.3062 0.3984 0.4983 0.6071 0.7259 0.8563 1.0000 |
0.0000 -2.2405 -2.6325 -2.5228 -2.3879 -2.2667 -1.7854 -1.4144 -1.0128 -0.8163 0.0001 |
0.0000 -5.7129 -9.1342 -11.6352 -13.6269 -15.0610 -15.4760 -15.0361 -13.1669 -9.1599 -0.0040 |
-0.0002 -2.5047 -3.1029 -3.1449 -3.1049 -3.0203 -2.5185 -2.0640 -1.5145 -1.1020 0.0000 |
0.0000 0.3106 1.9399 3.4312 4.4225 4.8813 5.2155 4.8620 3.9435 2.0862 -0.0007 |
0.0000 -3.7175 -5.3267 -6.1836 -6.7389 -7.0022 -6.5791 -5.8603 -4.6311 -3.0053 0.0000 |
0.0000 -4.0929 -5.9663 -6.9589 -7.5762 -7.8436 -7.3831 -6.5748 -5.2060 -3.3560 0.0000 |
1.0000 1.1249 1.2112 1.2807 1.3404 1.3861 1.3997 1.3853 1.3263 1.2118 1.0001 |
0.0000 0.1249 0.2112 0.2807 0.3404 0.3861 0.3997 0.3853 0.3263 0.2118 0.0001 |
Table 5: Percentage deviations and interaction parameters (α) for the system anisic aldehyde (AA) + methyl acetate (MA).
| X1 | %UNR | %Uimx | %UIR | %UR | %UJ | %UD | U2/U2imx | α |
|---|---|---|---|---|---|---|---|---|
| 303.15 K | ||||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
0.0000 -0.8558 -1.3063 -1.6326 -1.7716 -1.5582 -1.2655 -0.9916 -0.7025 -0.4157 0.0000 |
0.0000 -3.5452 -6.2727 -8.4566 -10.0142 -10.7478 -10.8191 -10.1658 -8.5096 -5.4363 0.0000 |
-0.0000 -0.4224 -0.5623 -0.6885 -0.7262 -0.4989 -0.2753 -0.1477 -0.0760 -0.0723 0.0000 |
0.0000 1.2459 2.3186 3.0674 3.6067 4.1013 4.2263 3.8464 2.9968 1.6792 -0.0000 |
0.0000 -2.4711 -4.2474 -5.6072 -6.4788 -6.6836 -6.4431 -5.7899 -4.6061 -2.7827 0.0000 |
0.0000 -2.6890 -4.5838 -6.0140 -6.9286 -7.1513 -6.8955 -6.1874 -4.9098 -2.9583 0.0000 |
1.0000 1.0749 1.1383 1.1933 1.2350 1.2553 1.2574 1.2391 1.1947 1.1183 1.0000 |
0.0000 0.0749 0.1383 0.1933 0.2350 0.2553 0.2574 0.2391 0.1947 0.1183 0.0000 |
| 308.15 K | ||||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
0.0000 -1.0772 -1.6420 -2.0093 -2.1373 -1.9203 -1.5766 -1.3072 -0.9482 -0.5661 0.0000 |
0.0000 -3.7667 -6.6029 -8.8233 -10.3695 -11.0998 -11.1262 -10.4789 -8.7606 -5.5966 0.0000 |
-0.0000 -0.6369 -0.8870 -1.0516 -1.0768 -0.8457 -0.5715 -0.4508 -0.3120 -0.2171 0.0000 |
-0.0000 1.0269 2.0434 2.8007 3.3616 3.8304 3.9614 3.5400 2.7563 1.5483 -0.0000 |
0.0000 -2.6979 -4.5899 -5.9921 -6.8558 -7.0596 -6.7725 -6.1241 -4.8714 -2.9485 0.0000 |
0.0000 -2.9162 -4.9450 -6.4325 -7.3417 -7.5541 -7.2377 -6.5234 -5.1753 -3.1290 0.0000 |
1.0000 1.0798 1.1464 1.2029 1.2448 1.2653 1.2661 1.2478 1.2013 1.1221 1.0000 |
0.0000 0.0798 0.1464 0.2029 0.2448 0.2653 0.2661 0.2478 0.2013 0.1221 0.0000 |
| 313.15 K | ||||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
0.0000 -1.5215 -2.1375 -2.5191 -2.5698 -2.3884 -2.0523 -1.6763 -1.2772 -0.8022 0.0000 |
0.0000 -4.1433 -7.1168 -9.4258 -10.9649 -11.7803 -11.8514 -11.1258 -9.3559 -6.0305 0.0000 |
-0.0000 -0.8907 -1.1839 -1.3644 -1.3208 -1.1426 -0.8995 -0.7007 -0.5567 -0.4085 -0.0000 |
-0.0000 0.7430 1.6492 2.3495 2.9814 3.4162 3.5314 3.1911 2.4104 1.2871 0.0000 |
0.0000 -3.0675 -5.0915 -6.5766 -7.4233 -7.7045 -7.4518 -6.7087 -5.3946 -3.3162 0.0000 |
0.0000 -3.3005 -5.4577 -7.0273 -7.9261 -8.2238 -7.9440 -7.1281 -5.7060 -3.4966 0.0000 |
1.0000 1.0885 1.1593 1.2191 1.2616 1.2850 1.2871 1.2662 1.2172 1.1325 1.0000 |
0.0000 0.0885 0.1593 0.2191 0.2616 0.2850 0.2871 0.2662 0.2172 0.1325 0.0000 |
| 318.15 K | ||||||||
| 0.0000 0.0832 0.1695 0.2592 0.3524 0.4494 0.5504 0.6557 0.7655 0.8802 1.0000 |
0.0000 -1.5703 -2.1584 -2.4387 -2.5311 -2.4840 -2.2147 -1.8838 -1.4956 -0.9862 0.0000 |
0.0000 -4.4409 -7.4434 -9.7058 -11.3224 -12.2863 -12.4291 -11.7342 -9.9300 -6.4634 0.0000 |
0.0000 -1.0403 -1.2390 -1.2685 -1.2342 -1.1728 -0.9904 -0.8410 -0.7218 -0.5616 0.0000 |
-0.0000 0.5781 1.7341 2.3660 3.0190 3.2598 3.3259 3.0291 2.3324 1.1190 -0.0000 |
0.0000 -3.3795 -5.4395 -6.8791 -7.8041 -8.2340 -8.0457 -7.3190 -5.9509 -3.7164 0.0000 |
0.0000 -3.6299 -5.8849 -7.3488 -8.3387 -8.7631 -8.5479 -7.7699 -6.3178 -3.9085 0.0000 |
1.0000 1.0951 1.1673 1.2265 1.2717 1.2998 1.3040 1.2836 1.2327 1.1430 1.0000 |
0.0001 0.0951 0.1673 0.2265 0.2717 0.2998 0.3040 0.2836 0.2327 0.1430 0.0000 |
Table 6: Percentage deviations and interaction parameters (α) for the system anisic aldehyde (AA)+ethyl acetate (EA).
| X1 | %UNR | %Uimx | %UIR | %UR | %UJ | %UD | U2/U2imx | α |
|---|---|---|---|---|---|---|---|---|
| 303.15 K | ||||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
0.0000 -1.4289 -2.3791 -3.0170 -3.3087 -3.3079 -2.9142 -2.4230 -1.7634 -1.0232 0.0000 |
0.0000 -2.3429 -4.0192 -5.2021 -5.8594 -6.0380 -5.6269 -4.8908 -3.7292 -2.1855 -0.0000 |
0.0000 -0.1118 -0.1928 -0.3247 -0.4069 -0.4443 -0.2958 -0.2356 -0.1677 -0.1621 0.0000 |
-0.0000 -0.2548 -0.3212 -0.3499 -0.2830 -0.1666 0.0963 0.1882 0.1818 0.0225 -0.0000 |
0.0000 -2.8249 -4.9026 -6.4078 -7.3061 -7.6356 -7.2717 -6.4492 -5.0299 -2.9970 -0.0000 |
0.0000 -2.9658 -5.1497 -6.7292 -7.6748 -8.0252 -7.6527 -6.7845 -5.2797 -3.1276 -0.0000 |
1.0000 1.0486 1.0855 1.1128 1.1284 1.1327 1.1228 1.1055 1.0790 1.0452 1.0000 |
0.0000 0.0486 0.0855 0.1128 0.1284 0.1327 0.1228 0.1055 0.0790 0.0452 0.0000 |
| 308.15 K | ||||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
0.0000 -1.5938 -2.6819 -3.3588 -3.6061 -3.6191 -3.1695 -2.6293 -1.9434 -1.0747 0.0000 |
0.0000 -2.5137 -4.3300 -5.5535 -6.1691 -6.3619 -5.8962 -5.1110 -3.9208 -2.2451 0.0000 |
0.0001 -0.2791 -0.5030 -0.6771 -0.7148 -0.7667 -0.5600 -0.4486 -0.3523 -0.2151 0.0000 |
-0.0000 -0.3339 -0.5570 -0.6485 -0.5041 -0.4076 -0.0863 0.0358 -0.0034 -0.0061 -0.0000 |
0.0000 -2.9900 -5.2018 -6.7428 -7.5967 -7.9381 -7.5199 -6.6501 -5.2058 -3.0480 0.0000 |
0.0000 -3.1620 -5.4764 -7.0859 -7.9980 -8.3589 -7.9322 -7.0100 -5.4599 -3.1891 0.0000 |
1.0000 1.0522 1.0926 1.1211 1.1358 1.1405 1.1292 1.1106 1.0833 1.0465 1.0000 |
0.0000 0.0522 0.0926 0.1211 0.1358 0.1405 0.1292 0.1106 0.0833 0.0465 0.0000 |
| 313.15 K | ||||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
0.0000 -1.8263 -2.9219 -3.6053 -3.8636 -3.8817 -3.4053 -2.7899 -2.1113 -1.1893 0.0000 |
0.0000 -2.8003 -4.6680 -5.9321 -6.5830 -6.7948 -6.3058 -5.4358 -4.2230 -2.4425 0.0000 |
-0.0003 -0.4575 -0.6553 -0.8182 -0.8615 -0.9226 -0.6994 -0.5287 -0.4628 -0.2988 -0.0001 |
0.0007 -0.5373 -0.8027 -0.8955 -0.7957 -0.6763 -0.3041 -0.0946 -0.1151 -0.0353 0.0000 |
0.0000 -3.2781 -5.5430 -7.1266 -8.0181 -8.3813 -7.9435 -6.9928 -5.5266 -3.2602 0.0000 |
0.0000 -3.4661 -5.8273 -7.4844 -8.4258 -8.8177 -8.3778 -7.3763 -5.8095 -3.4352 0.0000 |
1.0000 1.0584 1.1003 1.1301 1.1459 1.1511 1.1391 1.1183 1.0901 1.0507 1.0000 |
0.0000 0.0584 0.1003 0.1301 0.1459 0.1511 0.1391 0.1183 0.0901 0.0507 0.0000 |
| 318.15 K | ||||||||
| 0.0000 0.1087 0.2152 0.3198 0.4224 0.5231 0.6220 0.7191 0.8144 0.9080 1.0000 |
0.0000 -2.1149 -3.2443 -3.9533 -4.1839 -4.1812 -3.7488 -3.1524 -2.3372 -1.3147 0.0001 |
0.0000 -3.1667 -5.1314 -6.4706 -7.1315 -7.3452 -6.9046 -6.0378 -4.6501 -2.6936 0.0000 |
0.0004 -0.6397 -0.8062 -0.9601 -0.9631 -1.0097 -0.8536 -0.7361 -0.5751 -0.3630 0.0000 |
-0.0001 -0.8329 -1.0964 -1.2243 -1.1137 -0.9703 -0.6646 -0.4647 -0.2835 -0.1411 -0.0007 |
0.0000 -3.6625 -6.0400 -7.7125 -8.6268 -9.0027 -8.6200 -7.6748 -6.0293 -3.5650 0.0000 |
0.0000 -3.8547 -6.3444 -8.0901 -9.0503 -9.4559 -9.0640 -8.0692 -6.3402 -3.7521 0.0000 |
1.0000 1.0665 1.1111 1.1432 1.1595 1.1648 1.1538 1.1326 1.0999 1.0561 1.0000 |
0.0000 0.0665 0.1111 0.1432 0.1595 0.1648 0.1538 0.1326 0.0999 0.0561 0.0000 |
Table 7: Percentage deviations and interaction parameters (α) for the system anisic aldehyde (AA)+n- butyl acetate (BA).
| UNR | Uimx | UIR | UR | UJ | UD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | χ2 | APE | χ2 | APE | χ2 | APE | χ2 | APE | χ2 | APE | χ2 | APE | |
| 303.15K | 0.0540 | -0.4593 | 15.0119 | -9.1641 | 0.177 | -0.9788 | 1.6587 | 2.9929 | 2.1848 | -3.548 | 2.619 | -3.887 | |
| 308.15K | 0.1322 | -1.0055 | 15.1483 | -11.326 | 0.3277 | -1.6685 | 1.6024 | 3.5886 | 2.4167 | -3.203 | 2.982 | -4.168 | |
| AA+MA | 313.15K | 0.2604 | -1.4765 | 16.3347 | -11.809 | 0.5071 | -2.1004 | 1.4839 | 3.3931 | 2.9497 | -5.091 | 3.674 | -4.647 |
| 318.15K | 0.4111 | -1.8977 | 16.6921 | -12.002 | 0.6759 | -2.453 | 1.5679 | 3.4547 | 3.3385 | -5.449 | 4.198 | -5.000 | |
| 303.15K | 0.1657 | -1.1667 | 8.1575 | -8.2186 | 0.0215 | -0.3855 | 1.1165 | 3.0097 | 3.0092 | -5.012 | 3.448 | -4.393 | |
| AA + EA | 308.15K | 1.0216 | -1.1985 | 11.5004 | -6.9659 | 0.578 | -0.550 | 0.3626 | 2.2607 | 5.2842 | -4.356 | 5.873 | -4.660 |
| 313.15K | 0.4063 | 2.0366 | 9.6912 | -7.4359 | 0.1023 | -0.7698 | 0.2453 | 1.9599 | 3.9874 | -4.794 | 4.529 | -5.110 | |
| 318.15K | 0.4302 | -1.6148 | 10.4674 | -7.7956 | 0.1099 | -0.8245 | 0.6606 | 1.8874 | 4.5296 | -5.161 | 5.141 | -5.500 | |
| 303.15K | 0.7169 | -1.9605 | 2.4444 | -3.6268 | 0.009 | -0.2129 | 0.0058 | -0.081 | 3.9674 | -4.621 | 4.381 | -4.854 | |
| AA+BA | 308.15K | 0.8509 | -2.1524 | 2.6789 | -3.8274 | 0.0317 | -0.4106 | 0.015 | -0.228 | 4.2269 | -4.809 | 4.684 | -5.061 |
| 313.15K | 0.9794 | -2.3268 | 3.048 | -4.1077 | 0.0484 | -0.5187 | 0.0346 | -0.387 | 4.6957 | -5.097 | 5.202 | -5.366 | |
| 318.15K | 1.1637 | -2.5664 | 3.5891 | -4.5029 | 0.0678 | -0.6278 | 0.0737 | -0.618 | 5.4392 | -5.539 | 6.003 | -5.820 | |
Table 8: Values of Chi Square Test and Average Percentage Error for the evaluated velocities in the studied binary mixtures at different temperatures (303.15 K-308.15 K).
From the values of experimental and evaluated velocity values, it may be concluded that, the Nomoto’s relation, Van Deal ideal mixing relation and Impedance relation of ultrasound velocity have provided good results.Thus, the linearity of molar sound velocity and additivity of molar volumes, as suggested by Nomoto, Van Dael and Vangeel and Impedance relation in deriving the empirical relations (eqn 5, 6 and 7) have been truly observed in the aforementioned binary liquid mixtures. The success of Nomoto’s relation in predicting the experimental ultrasonic velocities for polar-polar liquid mixtures has also been emphasized by others [22].