
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review - (2022)Volume 12, Issue 2
Background: Postoperative fever is one of the common complications in neurosurgery; intracranial aseptic inflammation and infection are important incentive factors. Continuous drainage of CSF via Lumbar Drainage (LD) is often used in the treatment of postoperative intracranial infection or aseptic inflammation. Compared with the previously reported placement of the LD after the onset of meningitis symptoms, we designed this Randomized Controlled Trial (RCT) to evaluate the effectiveness and safety of early drainage (1st day post-operation) of Cerebrospinal Fluid (CSF) using the preset lumbar cistern to prevent delayed fever or reduce its treatment time after Cerebellopontine Angle (CPA) tumor surgery.
Methods: Patients suffering from CPA tumor and completed resection of the tumor with intraoperative dura opening time>4 h are being recruited for this study. The study is a 2-arm RCT to compare an intervention group receiving postoperative early LD and standard postoperative care to a control group receiving standard postoperative care only. Duration of fever in patients with delayed fever after operation, as the main outcome, will be contrasted in the two groups.
Discussion: Here, we present the study design of a prospective RCT to evaluate the safety and efficacy of using preoperative preset LD to treat or reduce of postoperative delayed fever.
Lumbar drainage; Cerebellopontine angle tumor; Randomized controlled trial; Delayed fever postneurosurgery; Infection; Aseptic inflammation
Postoperative fever is one of the common complications in neurosurgery [1,2]. Except for the absorption heat and other systemic factors such as hypostatic pneumonia, intracranial aseptic inflammation and infection are important incentive factors resulting delayed postoperative fever (fever occurred after the third day post-operation) [3-5]. Aseptic meningitis can be produced by bone foam, necrotic cell, hemostatic materials, repair materials or bloody Cerebrospinal Fluid (CSF) [6]. It is well known that infection is caused by bacterial invasion during surgical operation. Fever may increase anxiety, delay the recovery, significantly prolong the hospitalization days after operation, increase medical expenses, and even increase the risk of death and disability [7,8].
In the case of intracranial infection, intravenous antibiotics alone are not enough. Continuous LD of CSF can remove bacteria, toxins and necrotic substances. Therefore, it can quickly relieve the symptoms of meningeal irritation, alleviate intracranial infection and enhance the therapeutic effect in a short time [7,8]. For aseptic inflammation, such as SAH, early preventive lumbar cistern drainage can also reduce the incidence of complications such as fever before the appearance of fever signs [9-11]. Therefore, we speculate that early LD of infectious factors or aseptic inflammatory factors plays a positive role in the prevention and treatment of the two situations.
The surgery of CPA tumor which locating deeply and surrounding dense nerve and vascular need more meticulous and spend much time resulting in a significant increase of delayed postoperative fever. Coincidently, in order to reduce the traction of cerebellum during operation, lumbar cistern catheter is often preset before operation to release a certain amount of CSF intra-operation [12-14]. So we designed this RCT to evaluate the effectiveness and safety of early drainage (1st day post-operation) of cerebrospinal fluid using the preset lumbar cistern to prevent delayed fever or reduce its treatment time after CPA tumor surgery. The successful verification of this scheme may greatly optimize the peri-operative treatment process of patients with complex CPA tumors and other complex intracranial tumors, and has great economic and social benefits.
Study design
The study is a 2-arm RCT to compare an intervention group receiving postoperative early LD and standard postoperative care to a control group receiving standard postoperative care only. It is conducted by a study group treating at least 70 patients with CPA tumor per year. Data management and monitoring will be performed by the Resman. Patients suffering from CPA tumor and completed resection of the tumor with intraoperative dura opening time>4 h are being recruited for this study. All medical treatment is performed according to local guidelines and standard operating procedures.
Subject inclusion criteria
• Age: 18-65 years
• First surgery for tumor in the cerebellopontine angle and dura opening time>4 h
• Informed consent by the patient or his/her legal representative
Subject exclusion criteria
• Abnormal inflammatory indicators before surgery
• Patients with severe intracranial hypertension or hydrocephalus
• Patients with severe heart liver lung and kidney insufficiency
• Patient with severe lumbar hyperostosis or intervertebral disc herniation
• Patient is the researcher or his consanguinity who may have improper informed consent
• The attending doctor thinks it is inappropriate to participate in this trial
• All subjects have the right to withdraw from the trial at any time. During the trial, the researcher also has the right to terminate the trial of the subjects who meet the following conditions
• Withdraw from the informed consent (the subjects decide to withdraw for any reason)
• The dural opening time was less than 4 hours
• The researchers believe that clinical Adverse Events (AE), laboratory abnormalities or secondary diseases (other systemic infections) indicate that it is not in the best interests of the subjects to continue to participate in the trial;
• The compliance of the subjects was poor
• After participating in the trial, it is found that the subjects do not meet the inclusion criteria seriously, or the subjects are lost to follow-up
• Subjects were imprisoned or forced to be isolated for treatment of mental or physical diseases (such as infectious diseases)
• The ethics committee or regulatory body requests to terminate the trial
• Serious violation of the protocol during the test.
Interventions
Patients with tumors in CPA were routinely treated with LD and tumor resection. Before opening the dura, the LD is turned on to release a certain amount of CSF slowly, and then the tumor was removed according to the conventional procedure. If the dura opening time is more than 4 hours during the operation, these patients were randomly divided into the early LD group and no early LD group. The patients’ LD in no early LD group was removed after surgery, and early LD group reserved the LD.
In the no early LD group, the body temperature was monitored routinely. If there is no fever after 3rd day post-operation, the patients could be discharged excluding other system abnormalities after 6th day post-operation; if the body temperature is more than 37.4 after 3rd day post-operation, lumbar puncture should be performed to test CSF. If the number of cells and White Blood Cells (WBC) in CSF is increased and other systemic infections are excluded, lumbar puncture should be performed to release CSF or LD should be placed to continuously drain CSF. If the body temperature is more than 38, the WBC count in cerebrospinal fluid is increased and CSF glucose was less than 2.2 mmol/l or CSF glucose/serum glucose was less than or equal to 0.4, meropenem and vancomycin should be given. After treatment, if the body temperature is normal for 3 consecutive days, and the routine WBC count in cerebrospinal fluid is less than 300 *106/L, and the sugar is normal, the lumbar cistern can be removed, and the discharge standard can be reached after excluding other system abnormalities
In the early LD group, the LD was opened and cerebrospinal fluid was continuously drained at a rate of 5-10 ml/h after eliminating CT abnormality of first day post-surgery. The routine, biochemical and culture tests of CSF were performed every two days. At the same time, the body temperature was routinely monitored. If the body temperature was normal for 72 h after 3rd day post-operation, the routine WBC count in CSF is less than 300*106/L, and the CSF glucose was normal, the lumbar cistern could be removed, and the discharge standard could be reached after excluding other system abnormalities. If the body temperature is more than 37.4 degrees after 3rd day post-operation, the lumbar cistern drainage should be continued. If the body temperature is more than 38, the WBC count in cerebrospinal fluid is increased and CSF glucose was less than 2.2 mmol/l or CSF glucose/serum glucose was less than or equal to 0.4, meropenem and vancomycin should be given. After treatment, if the body temperature is normal for 3 consecutive days, and the routine WBC count in CSF is less than 300*106/L, and the CSF glucose is normal, the lumbar cistern can be removed, and the discharge standard can be reached after excluding other system abnormalities (Figure 1).
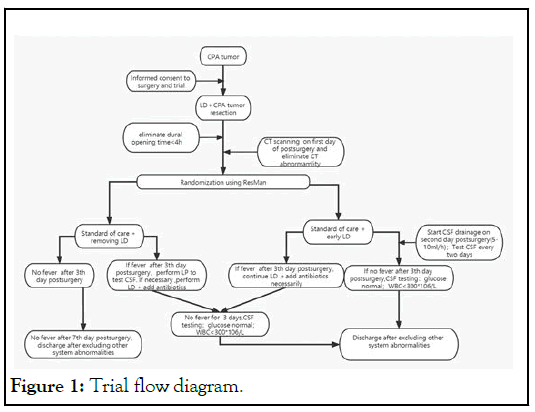
Figure 1: Trial flow diagram.
If the number of WBC in CSF gradually decreased and then increased during LD drainage, the possibility of retrograde infection should be considered, the LD should be removed and lumbar puncture should be performed according to the need. If the time of LD exceeds 10 days, LD repositioning is need in next lumbar segment or performs continuous lumbar puncture.
Consent to study participation
Consent to study inclusion is sought after explanation and agreement to CPA tumor surgery. Thus, patients capable of consenting to the CPA tumor surgery will be informed about the study details themselves and may or may not agree to participate. If a patient is incapable of consenting to the proposed treatment, the legal representative should be informed on the conditions of treatment choices and afterwards, on the details of the study. A patient may be randomized if the legal representative gives informed consent to the study.
Randomization
Any patient meeting the inclusion criteria and not violating the exclusion criteria may participate in the study and be randomized to either receive a postoperative early LD or not, thus defining the two distinct groups early LD and No early LD. Randomization is performed via a dedicated internet site accessible for investigator.
Sample size calculation
To test whether the early lumbar cistern drainage group is better than the non-early lumbar cistern drainage group. The two groups were divided into two groups according to the ratio of 1:1. According to the results of earlier review of dural opening time more than 4 hours, the descriptive statistical result of fever time in early lumbar cistern drainage group was 7.76 ± 13 days, the descriptive statistical result of the group without early lumbar cistern drainage was 11. 23 3 ± 93 days, the smaller the index value, the better the treatment effect. Set the threshold value of superiority to 1.5 (SM value), take α=0.05, β=20, the sample size required for this trial is N1=N2=56. If the loss of follow-up rate is 20%, 70 patients with N1=N2 should be included in each group. Therefore, 140 patients were included in the study.
Compliance with ethical standards
Informed consent was obtained from each participant in this study. Review and analysis of patient information were approved by the Ethical Committee of Tangdu Hospital. This RCT was registered with the Chinese Clinical Trial and its registration no. is ChiCTR2100049057.
Outcome access
The main outcome measures of this study were the duration of fever in patients with delayed fever after operation.
The secondary observation indexes of this study were fever rate after 3rd day post-operation, postoperative hospital stay, cerebrospinal fluid fistula, 30 day readmission rate, unplanned reoperation rate, lumbar cistern retrograde infection rate, lumbar cistern related complications and so on.
The following parameters will be recorded concerning primary and/or secondary outcome parameters
• Gender
• Age
• Dural opening time
• Antibiotic prophylaxis
• Drainage tube in the operation area (yes/no) and the time of extraction
• Duration of lumbar drainage
• Amount of CSF drained by lumbar drain (ml)
• CSF test results
• Blood test results
• Daily maximum body temperature
• Use of restricted antibiotics
• More detail in Case Report Form (CRF)
Adverse Events (AE) and Severe Adverse Events (SAE)
Definition of adverse events and severe adverse events
The term “Adverse Event” (AE) describes any sign symptom, syndrome or any disease
Occurring newly in a trial participant after consent to the trial
Being of particular interest for the assessment of the disease or the security of the therapeutic concept. In this trial AEs include
• Complications related to lumbar drainage amount,
• Complications related to insertion of a lumbar drainage,
• Any SAE
The term AE does not implicate a causal correlation with the participation in the trial. AEs are divided in Severe (SAE) and non-severe (AE) Adverse Events.
An SAE is any AE occurring during the trial that is related to
• Death
• Any life-threatening condition
• Re-hospitalisation or prolongation of hospitalization
• Long-term or severe restraint of the state of health
Statistical analysis
All data are described according to their mean, median or frequency, as applicable. Descriptive statistics of the no early LD group and early LD groups were used to compare all relevant patient characteristics. Continuous data with a normal distribution were statistically tested for group differences using the Student t-test. Data without normal distribution were analyzed using the Mann-Whitney U-test. Readmission, complication, and mortality rates were analyzed using the chisquare test or the Fisher exact test. A p value<0.05 was considered statistically significant.
Continuous drainage of CSF via LD is often used in the treatment of postoperative intracranial infection or aseptic inflammation [4,5,9]. Compared with the previously reported placement of the LD after the onset of meningitis symptoms, it is the first time that we propose a protocol which putting forward the concept of using early LD of CSF to treat postoperative delayed fever.
The literature reported that the infection rate after neurosurgery is 1%-11% and aseptic meningitis is more common after posterior fossa surgery with incidence up to 30% which lead to delayed postoperative fever [14,15-17]. Among all the feverrelated factors, the duration of operation is one of the most important factor [18,19]. In our previous clinical work, we found that compared with the whole operation time, dural opening time has a higher correlation with delayed fever. In our previous retrospective summary, we found that the delayed fever rate of complex intracranial tumors with dural opening time more than 4 hours after operation was about 30%, in which aseptic inflammation accounted for about 20%, infection accounted for about 10%, and early lumbar drainage of cerebrospinal fluid may reduce the delayed fever rate and treatment time to a certain extent. In order to further evaluate the therapeutic effect of early postoperative LD on postoperative delayed fever, we designed this prospective trial for patients with CPA tumor whose dural opening time is more than 4 h during surgery [20-25].
The use of CSF drainage via a lumbar drain during cranial surgery is a common practice for reducing ICP and enhancing exposure. Despite its common practice, the use of a lumbar drain is not without complications including headache, nausea, and vomiting; abducens palsy; intracranial hypotension; lumbar nerve root irritation; cerebellar tonsillar herniation. However, according to our experience, the related complications can be prevented and controlled through the joint efforts of doctors, patients' families and nurses.
This protocol aims at evaluating the safety and efficacy of using preoperative preset LD to treat or reduce of postoperative delayed fever. Moreover, in the case of encouraging results-in other words, early LD could significantly reduce the duration of delayed fever post-operation and length of stay, multi-center trial may be started.
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
Citation: Zhao T (2022) The Effect of Postoperative Early Lumbar Drainage on Delayed Fever after Cerebellopontine Angle Tumor Surgery: Study Protocol for a Randomized Controlled Trial. J Clin Trials. 12:493.
Received: 18-Mar-2022, Manuscript No. JCTR-22-14400; Editor assigned: 22-Mar-2022, Pre QC No. JCTR-22-14400(PQ); Reviewed: 05-Apr-2022, QC No. JCTR-22-14400; Revised: 12-Apr-2022, Manuscript No. JCTR-22-14400 (R); Published: 19-Apr-2022 , DOI: 10.35248/2167-0870.22.12.493
Copyright: © 2022 Zhao T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.