Emergency Medicine: Open Access
Open Access
ISSN: 2165-7548
ISSN: 2165-7548
Research Article - (2015) Volume 5, Issue 6
Background: It has been postulated that there exists a discrepancy of methicillin-resistant Staphylococcus aureus (MRSA) minimum inhibitory concentration (MIC) results from various measurement methods. The association between higher MRSA MIC (MIC ≥ 2 μg/ml) and worse clinical outcome has been previously reported. Therefore, it is clinically essential to investigate whether such a discrepancy exists between the different MIC measurement methods.
Methods: From November 2009 to March 2011, 55 MRSA isolates were prospectively obtained at two emergency departments in Japan. The MIC of the isolates were measured by Etest® and five broth microdilution (BMD) methods, namely Eiken®, MicroScan® prompt method, MicroScan® turbidity method, Phoenix® and Vitek2® system, respectively. The MIC results of vancomycin, teicoplanin, linezolid, daptomycin and quinupristin-dalfopristin (Q-D) were evaluated. Statistical analysis was performed using the Wilcoxon signed rank test and Bland-Altman’s analysis.
Results: There was a tendency of constant and significant discrepancy of MIC results of anti-MRSA antibiotics between Etest® and the BMD methods for MRSA isolates. The averages of the vancomycin MIC were 1.86 μg/ ml in Etest® and 0.74 μg/ml in the Phoenix® method (p<0.01), respectively. For teicoplanin, they were 1.86 μg/ml and 0.60 μg/ml (p<0.01), and for linezolid they were 2.55 μg/ml and 1.18 μg/ml (p<0.01) with respect to Etest® and the Phoenix® method, respectively. Among the BMD methods, however, the MicroScan® prompt method and the MicroScan® turbidity method had less discrepancy from Etest® for vancomycin MIC measurement.
Conclusion: The MIC measured by various BMD methods tended to show consistently lower results compared to those measured by Etest®. Among the BMD methods, however, the MicroScan® prompt method and the MicroScan® turbidity method had less discrepancy from Etest® for vancomycin MIC measurement.
Keywords: Methicillin-resistant Staphylococcus aureus, Minimal inhibitory concentration, Anti-MRSA antibiotics, Antibiogram, MIC creep, Broth microdilution methods, Etest®
Currently, MRSA infections have been increasing in number worldwide, including in Japan. The Japan Nosocomial Infections Surveillance report of 2011 showed the rate of MRSA in Staphylococcus aureus isolates was 53% [1].
In 2003, the increase in vancomycin MIC was reported to be an important socio-economic issue as well as a threat to the health care system [2]. In 2006, the Clinical Laboratory Standard Institutes (CLSI) changed the breakpoint of the vancomycin MIC for MRSA [3]. Currently, with the rise in the vancomycin MIC, the Infectious Diseases Society of America (IDSA) guidelines recommend setting a higher vancomycin trough level at 15~20 μg/mL [4]. A recent report showed that higher vancomycin MIC levels (MIC ≥ 2) were associated with higher mortality rates in MRSA bacteremic patients even with the use of specific antimicrobial agents against MRSA [5].
At the same time, higher vancomycin trough levels were reportedly associated with higher rates of adverse events, including ototoxicity and nephrotoxicity [6,7].
Therefore, it is essential to obtain MRSA MIC results when managing MRSA infections. Etest® is considered to be one of the standard measurement methods for determining the MIC for anti- MRSA antibiotics [8]. It was the method used in the prior studies, which showed the relationship between higher MIC and worse outcome [5].
In most clinical microbiology laboratories, however, BMDs are widely used because of their simplicity. It has been postulated that a discrepancy exists between the MRSA MIC results in various measurement methods, especially between Etest® and BMD methods [9-12]. Therefore, it is clinically essential to investigate whether such a discrepancy exists between these different MIC measurement methods. In our study, MIC results of MRSA clinical isolates were measured and analyzed using Etest® and five BMD methods for five anti-MRSA antibiotics including vancomycin, teicoplanin, linezolid, daptomycin and Q-D.
From November 2009 to March 2011, a prospective observational study was performed at two metropolitan tertiary Emergency Departments (EDs) in Japan [13]. Patients with high risk of MRSA colonization were determined a priori and MRSA surveillance cultures were obtained. Specimens for culture were obtained from the patients by nasal swab. The MRSA colonies were stored in deep freeze at -20°C. The 55 clinical MRSA isolates were then used to compare MIC results. Bacterial frozen stocks of the original clinical isolates were thawed in skimmed milk based broth and the same generation bacterial subcultures were then used for all MIC measurements. Etest® (SysmexbioMérieux Co., Tokyo, Japan) and five BMD methods: Eiken® (Eiken Chemical Co., Ltd. Japan), MicroScan® prompt method (Siemens Co., Ltd. CA, USA), MicroScan® turbidity method (Siemens Co., Ltd. CA, USA), Phoenix® (Becton, Dickinson and Company, NJ, USA), and Vitek2® system (Sysmex-bioMérieux Co., Tokyo, Japan) were analyzed. The MIC results of vancomycin, teicoplanin, linezolid, daptomycin and Q-D were also evaluated. The 2012 CLSI guidelines [3] were used for detection of antibiotic resistance. Table 1 shows the summary of the MIC measurement methods and their targeted anti-MRSA antibiotics. All available combinations were measured in our study.
| Targeted anti-MRSA antibiotics | ||||||
|---|---|---|---|---|---|---|
| MIC measurement methods | VAN | TEC | LZD | DAP | Q-D | |
| Etest | x | x | x | x | x | |
| Eiken® | x | x | x | |||
| BMD | MicroScan® (prompt method) | x | x | x | x | |
| MicroScan® (turbidity method) | x | x | x | x | ||
| Phoenix® | x | x | x | |||
| Vitek2® | x | x | x | x | ||
MIC=Minimum inhibitory concentration; MRSA=Methicillin-resistant Staphylococcus aureus; BMD=Broth microdilution; VAN=Vancomycin; TEC=Teicoplanin; LZD=Linezolid; DAP=Daptomycin; Q-D=Quinupristindalfopristin
Table 1:The summary of MIC measurement methods and their targeted anti- MRSA antibiotics.
Etest® was performed by evenly spreading the medium in three directions on a commercial agar plate (Mueller Hinton II Agar®, Becton, Dickinson and Company (BD), NJ, USA). The medium was prepared to achieve the bacterial concentration (0.5 McFarland) according to the turbidity standard technique. Etest® and the broth microdilution methods were performed by technicians unrelated to the study to thereby try and limit potential bias.
This study was approved by the Institutional Review Board of the St. Marianna University School of Medicine, Japan. The study design, data collection, data analysis, and manuscript preparation were conducted without industrial financial support.
The statistical analysis was performed using Wilcoxon signed rank test and Bland-Altman’s analysis [14]. Bland-Altman’s analysis was performed using International Business Machines (IBM) Statistical Package for the Social Sciences software version 22.0 (IBM, Corp., Armonk, NY, USA). Bland-Altman’s analysis provided the mean difference in addition to the 95% limits of agreement corresponding to the mean difference ±2 standard deviations (SD).
The summary of the MIC measurement methods and their targeted anti-MRSA antibiotics is summarized in Table 1. In our study, all the available combinations of MIC measurements, as summarized in Table 1, were performed on all 55 MRSA clinical isolates. MRSA isolates of the same generation of subculture were used for all MIC measurements. Etest® and the five BMD methods were performed to measure the MIC of vancomycin, teicoplanin and linezolid. The five BMD methods were as outlined in the methods above. With regard to daptomycin, Etest® and the Vitek2® method were performed. With regards to Q-D, Etest® and the MicroScan® turbidity method and the MicroScan® prompt method were performed.
The MIC results of vancomycin are shown in Table 2 and in Figure 1 (a-e in the left column), respectively. The distribution of the MIC results with modal and average MICs are respectively summarized in Table 2. The distribution of MIC results was constantly and significantly higher by Etest® compared to the BMD methods. The mode of vancomycin MIC was 2 μg/ml (74.5%, 41/55) in Etest® and 0.5 μg/ml (52.7%, 29/55) with the Phoenix® BMD method. The averages of vancomycin MIC were 1.86 μg/ml with Etest® and 0.74 μg/ml with Phoenix® BMD method (p<0.01).
| No. of isolates (%) with MIC (μg/ml) determined by: Broth microdilution methods | ||||||
|---|---|---|---|---|---|---|
| Vancomycin MIC (μg/ml) | Etest® | Eiken® | MicroScan® (prompt) | MicroScan® (turbidity) | Phoenix® | Vitek2® |
| 0.5 | 0(0) | 8(14.5) | 1(1.8) | 0(0) | 29(52.7) | 7(12.7) |
| 1 | 2(3.6) | 45(81.8) | 23(41.8) | 24(43.6) | 26(47.3) | 45(81.8) |
| 1.5 | 12(21.8) | - | - | - | - | - |
| 2 | 41(74.5) | 2(3.6) | 30(54.5) | 27(49.1) | 0(0) | 3(5.5) |
| 4 | 0(0) | 0(0) | 1(1.8) | 4(7.3) | 0(0) | 0(0) |
| Modal MIC (μg/ml) | 2 | 1 | 2 | 2 | 0.5 | 1 |
| MIC average (μg/ml) | 1.86 | 0.96 | 1.58 | 1.7 | 0.74 | 0.99 |
| Difference from Etest® | - | p<0.01 | p=0.02 | p=0.05 | p<0.01 | p<0.01 |
MRSA=Methicillin-resistant Staphylococcus aureus; MIC=Minimum inhibitory concentration
Table 2:Comparison of vancomycin MICs in MRSA determined by Etest® and five broth microdilution methods.
Figure 1 (a-e in the left column) shows the scatterplot for the distribution of vancomycin MICs as determined by Etest® and those determined by the five BMD methods. The vertical axes are the MIC results by Etest® and the horizontal axes are those from each BMD method. The numbers on each cell are the numbers of isolates which showed the combination of measurement results. For example in the Etest® and Phoenix® scatterplot (Figure 1d), there were 15 isolates which resulted in a MIC of 2.0 μg/ml by Etest® and a MIC of 0.5 μg/ml by the Phoenix® method, respectively. The cells on the diagonal line (shaded with gray) correlate completely with the MIC measurements by Etest® and the BMD method. The cells on left upper triangle suggest that Etest® indicated higher MICs. The cells located furthest from the diagonal line showed a larger discrepancy of MICs between the two measurement methods. As can be visualized in the scatterplots, the MICs were constantly and significantly higher by Etest®, and this tendency was more prominent in the Eiken®, Phoenix® and Vitek2® BMD methods. Vancomycin MICs measured by Etest® were consistently twofold dilutions higher than the MICs determined by Eiken®, Phoenix® and Vitek2® BMD methods. The discrepancy was less prominent with the MicroScan® prompt method and the MicroScan® turbidity method; however, the MICs of those methods were still persistently higher in Etest®. Lower MICs by the BMD methods have a risk of underestimating the MRSA MIC, which poses a risk of targeting an insufficiently low trough level of anti-MRSA antibiotics when the MIC measured by Etest® is truly higher to warrant targeting a higher trough level. The difference of the discrepancy is also demonstrated with Bland-Altman’s plot in Figure 2. The plot confirmed a larger discrepancy with the Eiken®, Phoenix® and Vitek2® BMD methods, and smaller discrepancies with the MicroScan® prompt method and the MicroScan® turbidity method.
Figure 2: Bland-Altman’s plot is shown to compare the extent of discrepancy between Etest® and each five BMDs. The five plots on the top row (A) show the vancomycin MIC discrepancies. The five plots on the bottom row (B) show the teicoplanin MIC discrepancies. The horizontal axis shows the mean of MRSA MICs (μg/ml) measured by Etest® and a BMD: example, the average of the MIC by Etest® and MIC by Eiken®. The vertical axis shows the difference of MIC measurement (μg/ml) obtained by Etest® and a BMD: example, MIC by Etest® minus MIC by Eiken®. The solid line indicates the average of the difference. The dotted lines indicate the ± 2 SD. The larger discrepancies in Eiken®, Phoenix® and Vitek2® and smaller discrepancies in MicroScan® prompt method and MicroScan® turbidity method are visualized. Note: MIC=Minimum inhibitory concentration; BMD=Broth microdilution; VAN=Vancomycin; TEC=Teicoplanin; SD=Standard deviation.
The MIC results of teicoplanin are summarized in Table 3 and in Figure 1 (f-j in the right column). The modal MICs were 2 μg/ml by Etest® and 0.5 μg/ml by the Phoenix® BMD method, respectively. The MIC averages were 1.86 g/ml by Etest® and 0.60 μg/ml by the Phoenix® (p<0.01) method. Teicoplanin MICs showed a similar tendency as those of vancomycin. The MICs generated by Etest® were consistently more than twofold dilutions higher than MICs measured by the Eiken® and Phoenix® BMD methods. The MIC values by Etest® were higher and paralleled those by the BMD methods, i.e. isolates with higher MIC values with Etest® also had higher MIC values for the Eiken® or Phoenix® methods as shown in Fig 1-f and 1-i. When measured by the Vitek2® method, 54 of 55 isolates resulted in MIC ≤ 0.5 μg/ml when the MIC by Etest® distributed from 0.75 μg/ml to 4 μg/ml with a modal MIC of 2 μg/ml, which indicated that the Vitek2® method could not provide a meaningful differentiation of MICs (Fig 1-j). A similar tendency was observed in the MicroScan® turbidity method and the MicroScan® prompt method; the teicoplanin MICs were almost all reported as ≤ 2 μg/ml by the MicroScan® turbidity method and the MicroScan® prompt method and could not differentiate regarding MICs lower than 2 μg/ml (Figure 1g and h).
| No. of isolates (%) with MIC (μg/ml) determined by: Broth microdilution methods | ||||||
|---|---|---|---|---|---|---|
| Teicoplanin MIC (μg/ml) | Etest® | Eiken® | MicroScan® (prompt) | MicroScan® (prompt) | Phoenix® | Vitek2® |
| ≤0.5 | 0(0) | 36(65.5) | - | - | 48(87.3) | 54(98.2) |
| 0.75 | 2(3.6) | - | - | - | - | - |
| 1 | 9(16.4) | 10(18.2) | - | - | 5(9.1) | 5(9.1) |
| 1.5 | 11(20.0) | - | - | - | - | - |
| 2 | 26(47.3) | 6(10.9) | (≤2) 53(96.4) | (≤2) 55(100) | 2(3.6) | 0(0) |
| 3 | 5(9.1) | - | - | - | - | - |
| 4 | 2(3.6) | 3(5.5) | 2(3.6) | 0(0) | 0(0) | 0(0) |
| Modal MIC (μg/ml) | 2 | 0.5 | ≤2 | ≤2 | 0.5 | 0.5 |
| MIC average (μg/ml) | 1.86 | 0.94 | 2.07 | 2 | 0.6 | 0.51 |
| Difference from Etest® | - | p<0.01 | p=0.03 | p=0.13 | p<0.01 | p<0.01 |
MRSA=Methicillin-resistant Staphylococcus aureus, MIC=Minimum inhibitory concentration
Table 3: Comparison of teicoplanin MICs in MRSA determined by Etest® and five broth micro dilution methods.
The MIC results of linezolid showed a similar tendency of higher MIC distribution by Etest® and lower distribution by the BMDs methods (Table S1 and Figure S1). The modal MICs were 3 μg/ml by Etest® and 1 μg/ml by the Phoenix® method, respectively. The MIC averages were 2.55 μg/ml by Etest® and 1.18 μg/ml by the Phoenix® method (p<0.01).
The MIC results of Q-D showed a similar tendency of higher MIC distribution by Etest® and a lower distribution by two BMD methods (Table S2 and Figure S2). The modal MICs were 0.75 μg/ml by Etest® and ≤ 0.5 μg/ml by the MicroScan® turbidity method. The MIC averages were 0.73 μg/ml by Etest® and 0.56 μg/ml by the MicroScan® turbidity method (p<0.01).
The MIC results of daptomycin by Etest® and the Vitek2® method are summarized in Table 4 and Figure 3. In contrast to the results of the other anti-MRSA antibiotics, only daptomycin MICs were distributed higher by the Vitek2® method than by Etest®.
| Daptomycin MIC (μg/ml) | No. of isolates (%) with MIC (μg/ml) determined by:BMD | |
|---|---|---|
| Etest® | Vitek2® | |
| 0.25 | 6(10.9) | 11(20.0) |
| 0.38 | 25(45.5) | - |
| 0.5 | 18(32.7) | 31(56.4) |
| 0.75 | 6(10.9) | - |
| 1 | 0(0) | 13(23.6) |
| Modal MIC (μg/ml) | 0.38 | 0.5 |
| MIC average (μg/ml) | 0.45 | 0.57 |
| Difference from Etest® | - | p<0.01 |
MRSA=Methicillin-resistant Staphylococcus aureus MIC=Minimum inhibitory concentration; BMD=Broth microdilution
Table 4:Comparison of daptomycin MICs in MRSA determined by Etest® and one broth microdilution method.
Our study has three clinical implications. First, by simultaneously evaluating all available combinations (Table 1), our study has shown that MICs, not only by vancomycin but also by other anti-MRSA antibiotics, had a similar tendency of higher MICs with Etest® compared to the BMD methods. Although similar previous studies have been published, to the best of our knowledge, our study is the first report in which Etest® and five BMD methods have been measured at one time for five anti- MRSA antibiotics (vancomycin, teicoplanin, linezolid, daptomycin and Q-D).
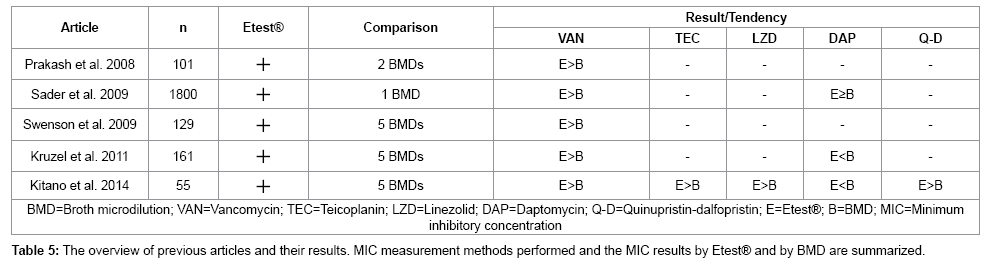
The previous articles are summarized in Table 5. Sader et al. [9] compared Etest® and one BMD method (CLSI being the reference BMD method) for vancomycin and daptomycin; they reported that vancomycin MICs obtained by Etest® were consistently higher than those obtained by the BMD method. Swenson et al. [10] compared Etest® and five BMD methods (MicroScan® prompt method, Vitek2®, Vitek Legacy®, Sensititre®, and Phoenix®) for vancomycin, which showed the similar tendency that the MicroScan® prompt method had a smaller discrepancy and that Vitek2® had a larger discrepancy to Etest®. Kruzel et al. [11] investigated Etest® and five BMD methods (Trek®, BMD in-house, Vitek2®, MicroScan®, and Phoenix®) for vancomycin and daptomycin, which showed a similar tendency of the MIC results by the Vitek2® and Phoenix® methods, which were distributed in a lower range compared to the MicroScan® method. Prakash et al. [12] investigated two kinds of Etest® and two BMD methods for vancomycin. These articles provided information comparing one or two pairs (i.e. BMDs vs. Etest® for vancomycin alone or for vancomycin/daptomycin). However, the information from our study have clinical importance in providing a more thorough perspective of the discrepancy by obtaining all the available combinations of five BMD methods and Etest® on anti-MRSA antibiotics all at one time. When measured by the Phoenix® method, the MIC measurements of vancomycin, teicoplanin and linezolid are all distributed lower than Etest® as previous studies have also demonstrated. Our study has provided further information that with respect to teicoplanin and linezolid, the discrepancies were larger than with vancomycin, the information that only our study could provide by simultaneous measurements and comparison.
Second, our study demonstrated that among the various BMD methods, the Eiken®, Phoenix® and Vitek2® methods had larger discrepancies compared to Etest®; the MicroScan® prompt method and the MicroScan® turbidity method had smaller discrepancies when compared to Etest® in respect of vancomycin MIC measurement. This provides important information in assessing MIC results obtained in the clinical setting. If the vancomycin MIC is measured by the Eiken®, Phoenix® or Vitek2® BMD methods, clinicians should be aware that it may considerably underestimate the MIC result. Conversely, if the vancomycin MIC is measured by the MicroScan® prompt method or the MicroScan® turbidity method, it can be assumed that it correlates fairly well with the MIC results obtained by Etest®.
Third, our results have shown that daptomycin MIC results were distributed lower by Etest® than by the Vitek2® method, which is the only reverse correlation among other anti-MRSA antibiotics. Previous studies have showed mixed results regarding the MIC of daptomycin. Sader et al. [9] compared Etest® and the CLSI the reference BMD method to daptomycin, which showed the BMD with a slightly lower MIC than by Etest®. Kruzel et al. [11] compared Etest® and the Trek® and MicroScan® BMD methods to daptomycin, which showed that both BMD methods had significantly higher MIC results compared to Etest®. Our results have shown that daptomycin MICs by various BMD methods are distributed higher than by Etest®. Although it requires further study to confirm this correlation, the information from our study has credibility given that the MICs of other anti-MRSA antibiotics measured at the same time had the opposite correlations.
Our study has several limitations. First, the 55 clinical isolates investigated were MRSA colonies from patients’ nares on admission to the ED. However, our study has generalization as in measuring the MIC level, materials from colonized strains and strains from clinical infections are not intrinsically different. Also, MRSA colonization in nares was reported to be associated with an increased risk of MRSA infection, which indicates that MRSA colonized strains are strongly associated with MRSA infections [15]. Second, the MRSA isolates were from two metropolitan tertiary EDs in Japan. The MRSA epidemiology or antibiogram might differ among institutions or geographic regions. However, it does not affect the MIC measurement methods as stated above. Third, MRSA-MIC measurements were performed only on one occasion. We optimized the MIC measurement accuracy by measuring all 55 samples at one time. After thawing and inoculating from the frozen MRSA colonies, the MRSA isolates of the identical generation of subculture were used for all MIC measurements (Etest® and five BMD methods) to minimize the measurement bias. The MIC of the same 55 colonies were previously measured with three BMD methods (Phoenix®, MicroScan® prompt and MicroScan® turbidity) for another study (data not published). There was minimal deviation between the two MIC results obtained by the same BMD methods at different times.
The MIC measured by various BMD methods tended to show lower results compared to those measured by Etest® with respect to anti- MRSA antibiotics (vancomycin, teicoplanin, linezolid and Q-D).
Among the BMD methods, the MicroScan® prompt method and the MicroScan® turbidity method had less discrepancies from Etest® compared to the Eiken®, Phoenix® and Vitek2® methods in vancomycin MIC measurement.
We are particularly grateful to Ms. Aiko Hosoyama who made significant contributions to this research project with her excellent administrative and technical support. Financial disclosure (COI): none reported. This study was conducted without industrial financial support.