Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2022)Volume 13, Issue 4
Purpose: To demonstrate the differences between visual evoked potential parameter values in recurrent optic neuritis in younger patients suffering from demyelinating diseases.
Design: Retrospective observational study-case series.
Methods: 18 patients aged 14-36 diagnosed with optic neuritis. Each patient underwent an eye examination, VEP test, neurologic examination and an adequate corticosteroid therapy, both in acute and recurrent optic neuritis.
Results: A statistically significant difference was observed in all subjects with regard to the values of visual acuity, amplitude and latency of the P100 wave in acute and recurrent optic neuritis (p<0.001). We also found a statistically significant spread between the eyes in recurrent optic neuritis (p=0.01). The mean value of visual acuity of the unaffected eyes in the first and second ON attack amounted to 1, while the mean value of visual acuity of the affected eyes amounted to 0.4 in the first attack and 0.1 in the second. The peak amplitude of the unaffected eyes was 15 μV in the first attack and 14.8 μV in the second. The amplitude of the affected eyes was 5.53 μV in the first attack and 2.92 μV in the second. The arithmetic mean latency of unaffected eyes was 101.2 ms in the first attack, while it amounted to 101.5 ms in the second. The latency value of affected eyes amounted to 120.5 ms in the first attack and 130.5 ms in the second. The decrease in visual acuity, amplitude reduction and the prolongation of P100 latency were statistically significantly higher in recurrent optic neuritis (p<0.001). The mean value of visual acuity of the affected eyes was lower by 0.3 in the second attack than in the first (z=3.86, p<0.001). The average amplitude of the affected eyes in the second attack was lower by 2.9 relative to the first (t=27.4; p<0.001). The average latency value of the affected eyes increased during the second attack relative to the first attack, namely by 9.9 (t=18.7, p<0.01).
Conclusion: The decrease in visual acuity, amplitude reduction and the prolongation of P100 latency in recurrent optic neuritis in patients with demyelinating disease are significant compared to the parameter values in acute optic neuritis.
Optic neuritis; Multiple sclerosis; Visual evoked potential
Optic Neuritis (ON) is a general term for optic neuropathy of idiopathic, inflammatory, infectious, or demyelinating etiology [1].
It is an acute inflammatory disorder of the optic nerve characterized by a sudden unilateral vision loss usually preceded by periocular or retrobulbar pain that intensifies with the movements of the eye. It is considered to be the most common cause of unilateral vision loss or visual impairment in young people [2].
ON most commonly affects adults between the ages of 20 and 45. Women are more prone to suffer from ON than men. About 20%- 40% of patients with optic neuritis develop multiple sclerosis [3].
The diagnosis of ON is primarily clinical. It includes simple procedures that can be performed in an outpatient setting, such as the examination of medical history, visual acuity, color vision and pupillary light reflex. Other, more complex procedures include visual field tests and visual evoked potentials. Laboratory findings are also used, while lumbar puncture is not necessary in typical cases of ON. While CT falls short when it comes to diagnosing ON, MR is a very strong predictor of Multiple Sclerosis (MS). However, one must bear in mind the predictions and treatment of further neurological events in MS [4,5].
The treatment depends on the underlying condition. Retrobulbar ON followed by a severe vision loss can be treated with high doses of steroids, i.e. 1000 mg of prednisolone a day for three days intravenously, and 1 mg of prednisolone per kg of body weight from the fourth to the fourteenth day orally. However, the only result of this treatment is faster vision recovery. The final visual acuity is the same with or without any high-dose steroid therapy after the period of one year. Nevertheless, this therapy prevents the relapse of multiple sclerosis [3].
When it comes to patients with typical ON, visual prognosis is good. Recovery can start very quickly, in a matter of days or weeks, while further recovery takes a slower pace over months to follow. Patients with normalized visual acuity may experience significant optic nerve dysfunctions, which can be proven by testing the color vision, visual field, and VEP. The visual field of patients whose visual acuity has returned to normal or near normal is often fully recovered when tested in photopia. Unfortunately, such patients may continue to suffer from an abnormally rapid loss of the focal visual stimulus observation and an abnormally rapid sensory fatigue for a long time. Patients who have had a typical ON attack may live to see a recurrence of the attack on the same or the other eye after a while [4,5].
The purpose of the research was to do the following with regard to the patients with acute and recurrent episodes of optic neuritis:
1. Examine and compare visual acuity of the healthy and affected eye upon the first optic neuritis attack.
2. Examine and compare the values of amplitudes and latencies of the P100 wave of the healthy and affected eye upon the first optic neuritis attack.
3. Examine and compare the visual acuity of the healthy and affected eye following a recurrent optic neuritis attack.
4. Examine and compare the values of amplitudes and latencies of the P100 wave of the healthy and affected eye following a recurrent optic neuritis attack.
The research involved 18 subjects aged 14 to 36 who were diagnosed with Optic Neuritis (ON) in the period from January 1st, 2009 to December 31st, 2015. A complete neurological examination was performed on all subjects. Visible evoked potential tests were carried out along with complete eye examinations. VEP tests were done upon the first optic neuritis attack and following recurrent attacks.
Visual Evoked Potentials (VEP) to a monocular stimulation of samples was recorded in accordance with the guidelines of the International Society for Clinical Electrophysiology of Vision (ISCEV guidelines).
Transient VEPs were taken on a Tomey EP-1000 (TOMEY GmbH Am Weichselgarten Erlangen, Germany). VEPs were induced using 1 checkerboard pattern stimulation and a presentation rate of 2 changes per second. Standard imaging conditions were also used, as recommended by ISCEV.
VEPs were detected by placing silver skin electrodes on the cortical projection of the visual sphere. The electrode placement was performed in accordance with the international 10–20 system, with the active electrode placed in the Oz position, and the reference electrode in the Fz position. Grounding was achieved by placing an electrode on the earlobe. The electrodes placed on the skin were previously cleaned with abrasive paste (Nuprep). Fixing the electrodes onto clean skin was carried out by filling the electrode disk with conductive paste (Ten 20 conduction). The number of average passes was 64, and at least two series were performed. Latency and amplitude values of the P100 wave were sent for analysis. VEP responses were successfully recorded in all 18 subjects (36 eyes).
Research location
The research was conducted at the Eye Clinic (the Outpatient Department for Electrophysiology of the Eye) of the University Hospital Center Split.
Research management
The research was retrospective in nature. It was qualitative in structure, and descriptive in terms of intervention and data processing.
Research description
Data sources consisted of the written protocol of the Eye Clinic, the Clinic of Neurology and the Clinic of Pediatrics of the University Hospital Center Split and the archives of medical history charts. Patients who met the criteria and whose data could be found in the written protocol but not in the archives were excluded from the research. The following patient parameters were analyzed: age, sex, visual acuity, and VEP tests monitoring the values of amplitude and latency of the P100 wave upon the first and second optic neuritis attacks.
Data collection methods
All data were statistically processed and presented using tables. Descriptive and inferential statistics methods were used in the analysis. Absolute values and percentages were used to describe the categorical data. Mean values and the interquartile range were used for numerical data deviating from the normal. For data following the normal distribution, we used the mean ± SD (95% CI). To compare the data, we turned to the Independent Samples t-Test, Dependent Samples t-Test, Mann-Whitney U-test and Wilcoxon signed-rank test. We interpreted the results at the significance level p<0.05. Statistical analysis of the data was performed using the MedCalc statistical software package for the Windows 10 computer interface (MedCalc software, Mariakerk, Belgium; version 11.5.1.0).
We analyzed the data on a sample of 18 subjects aged 14 to 36 diagnosed with Optic Neuritis (ON) in the period from 2009 to 2015 at the Outpatient Department for Electrophysiology of the Eye of the University Hospital Center Split. Table 1 shows the demographic data of subjects with optic neuritis.
| Sex; n | Men | 5 |
|---|---|---|
| Women | 13 | |
| Age upon the 1st attack (years) | Mean value (Q1-Q3; min-max) | 30 (22-34; 14-36) |
| Age upon the 2nd attack (years) | Mean value (Q1-Q3; min-max) | 32.5 (23-36; 16-39) |
| Age difference of subjects between the first and second attack (years) | Mean value (Q1-Q3; min-max) | 2 (1.7-2; 1-3) |
Table 1: Demographic data of subjects with optic neuritis.
The right eye was affected in six subjects, while the left eye was affected in twelve subjects. Our research of recurrent attacks has shown that ON affected the same eye that was affected in the first attack. We studied three parameters: visual acuity, amplitude, and latency of the P100 wave. Within the presented sample, we have found that all subjects with optic neuritis experienced a deterioration of all three parameters in the affected eyes.
Visual acuity
Table 2 shows the visual acuity of the unaffected and affected eyes and the comparison between the two with regard to each attack.
| Unaffected eye | Affected eye | Pa | |
|---|---|---|---|
| 1st attack | 1 | 0.4 (0.4-0.5; 0.3-0.5) | <0.001 |
| 2nd attack | 1 | 0.1 (0.1-0.2; 0.1-0.3) | <0.001 |
Note: a: Mann-Whitney U Test
Table 2: Mean value (Q1-Q3; min-max) of visual acuity of the unaffected and affected eyes respectively upon each attack.
Visual acuity of the unaffected eyes upon the 1st and 2nd attack was significantly higher compared to that of the affected eyes (P<0.001) (Table 2).
Table 3 shows the comparison of visual acuity of affected eyes between the 1st and 2nd attack.
| Attack | |||
|---|---|---|---|
| Visual acuity | 1st | 2nd | Pb |
| Mean value (Q1-Q3; min-max) Affected eye | 0.4 (0.4-0.5; 0.3-0.5) | 0.1 (0.1-0.2; 0.1-0.3) | <0.001 |
Note: b: Wilcoxon signed-rank test
Table 3: Comparison of visual acuity of affected eyes between the 1st and 2nd attack.
The mean value of visual acuity of the affected eye was lower by 0.3 in the 2nd attack compared to the 1st attack (z=3.86; p<0.001).
All 18 subjects experienced vision impairment in the affected eye. The spread was 0.3 (Q1-Q3:0.27-0.3; min-max: 0.2-0.4).
Figure 1 shows graphical representation of the mean value (Q1- Q3; min-max) of visual acuity of the eye affected by optic neuritis during the 1st and 2nd attacks.
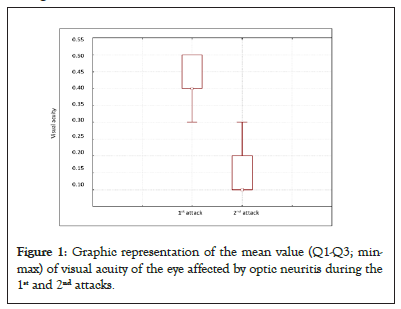
Figure 1: Graphic representation of the mean value (Q1-Q3; minmax) of visual acuity of the eye affected by optic neuritis during the 1st and 2nd attacks.
While examining visual acuity, we have come to know that it remained the same in the unaffected eyes. On the other hand, there was a decrease in visual acuity in the eyes affected by optic neuritis attacks (Table 2). During the 1st attack, the visual acuity of the unaffected eye remained the same, while there was a decrease in visual acuity of the affected eye. We have also noticed that visual acuity of the unaffected eye was not impacted by the 2nd optic neuritis attack, but there was a greater decrease in visual acuity of the affected eye than during the 1st attack (Table 2). By comparing visual acuity of the affected eyes between the 1st and 2nd attack, we have come to the conclusion that there was a statistically significant decrease, as well as that visual acuity upon the 2nd attack was worse by 0.3 than that upon the 1st attack (Table 3).
Amplitude
Table 4 shows the amplitude of the unaffected and affected eye, as well as the comparison between the two with regard to each attack.
| Unaffected eye | Affected eye | Spread in the 1st and 2nd attack | Pc | |
|---|---|---|---|---|
| 1st attack | 15.0 ± 1.87 | 5.53 ± 0.75 | 9.0 (95%CI: 7.6-10.4) | <0.001 |
| 2nd attack | 14.8 ± 2.04 | 2.92 ± 0.73 | 11.2 (95%CI: 9.5-13) | <0.001 |
Note: c: Independent samples t Test
Table 4: Arithmetic mean ± SD amplitude of the unaffected and affected eye and the spread in each attack.
The value of the amplitude of the unaffected eye is significantly higher compared to the affected eye in patients upon the 1st attack (t=12.7; p<0.001). The spread in amplitude values of the unaffected and affected eye amounts to 9.0 (95 CI: 7.6-10.4). The value of the amplitude of the unaffected eye is significantly higher compared to the affected eye in patients upon the 2nd attack (t=13.2, p<0.001). The spread in amplitude values of the unaffected and affected eye amounts to 11.2 (95 CI: 9.5-13).
Figure 2 shows graphical representation of the arithmetic mean ± SD of the affected eye amplitude during the 1st and 2nd attack.
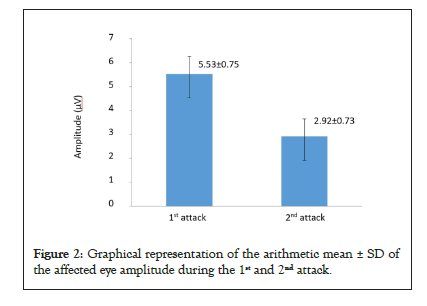
Figure 2: Graphical representation of the arithmetic mean ± SD of the affected eye amplitude during the 1st and 2nd attack.
Table 5 displays the comparison of the amplitude between the 1st and 2nd optic neuritis attack.
| 1st attack | 2nd attack | Spread in 1st and 2nd attack | Pd | ||
|---|---|---|---|---|---|
| Amplitude | Unaffected eye | 15.0 ± 1.87 | 14.8 ± 2.04 | 0.206 ± 0.36 (95%CI: 0.03-0.4) | 0.026 |
| Affected eye | 5.53 ± 0.75 | 2.92 ± 0.73 | 2.6 ± 0.4 (95%CI: 2.4-2.8) | <0.001 | |
Note: d: Dependent samples t Test
Table 5: Display of the amplitude comparison between the 1st and 2nd optic neuritis attack.
Although we have proven a statistically significant spread in the amplitude of the unaffected eye between the 1st and 2nd attack, that has no clinical significance (t=2.44; p=0.026).
The mean value of the amplitude of the affected eye in the 2nd attack was lower by 2.9 than in the 1st attack (t=27.4; p<0.001).
By examining the amplitude of the P100 wave, we found that there was no significant decrease in the amplitude in the eyes not affected by optic neuritis, while in the affected eyes; there was a decrease in the amplitude that is both statistically and clinically significant. During the 1st attack, the amplitude of the affected eye was reduced in relation to the amplitude of the unaffected eye. During the 2nd optic neuritis attack, there was a small decrease in the amplitude in the unaffected eyes, while the decrease in the amplitude of the affected eyes was significant (Table 4). Comparing the decrease in the amplitude between the 1st and 2nd optic neuritis attack, it has come to our attention that there is statistically significant spread in both the unaffected and affected eyes. There is no clinically significant spread in the unaffected eyes, while in the affected eyes; it amounts to 2.6 μV (Table 5).
Latency
Table 6 shows the latency of the unaffected and affected eye, as well as the comparison with regard to each attack.
| Unaffected eye | Affected eye | Spread in 1st and 2nd attack | Pc | |
|---|---|---|---|---|
| 1st attack | 101.2 ± 2.16 | 120.5 ± 0.73 | 18.4 (95CI: 15.9-20.8) | <0.001 |
| 2nd attack | 101.5 ± 1.96 | 130.5 ± 2.4 | 27.7 (95CI 24.3-30.0) | <0.001 |
Note: c: Independent samples t Test
Table 6: Arithmetic mean ± SD latency of the unaffected and affected eye and the spread with regard to each attack.
The latency was significantly longer in the affected eye compared to the unaffected eye of the patients in the 1st attack (t=15.31; p<0.001). The spread in average latency values of the unaffected and affected eye amounted to 18.4 (95 CI: 15.9-20.8).
There is a statistically significant spread in latency values between the unaffected and affected eye in patients in the 2nd attack (t=16.4; p<0.001). The spread in average latency values of the unaffected and affected eye amounts to 27.7 (95 CI: 24.3-31.0).
Figure 3 shows graphical representation of the arithmetic mean ± SD latency of the affected eye during the 1st and 2nd optic neuritis attack.
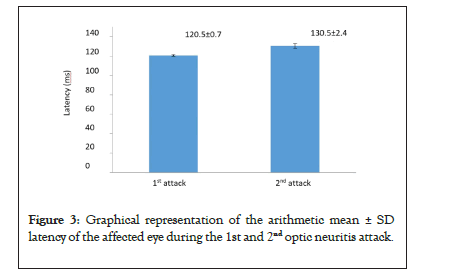
Figure 3: Graphical representation of the arithmetic mean ± SD latency of the affected eye during the 1st and 2nd optic neuritis attack.
Table 7 shows the comparison of latency between the 1st and 2nd optic neuritis attack.
| 1st attack | 2nd attack | Spread in 1st and 2nd attack | Pd | ||
|---|---|---|---|---|---|
| Amplitude | Unaffected eye | 101.2 ± 2.16 | 101.5 ± 1.96 | 0.27 ± 0.6 (95%CI: -0.05 to 0.58) | 0.001 |
| Affected eye | 120.5 ± 0.73 | 130.5 ± 2.4 | 9.9 ± 2.3 (95%CI: -11 to -9) | <0.001 | |
Note: d: Dependent samples t Test
Table 7: Display of the comparison of latency between the 1st and 2nd optic neuritis attack.
By analyzing the latency of the P100 wave, we have noticed that there were no changes in latency in the unaffected eyes, while there was a prolongation of latency in the eyes affected by an optic neuritis attack. During the 1st attack, the latency of the affected eye was prolonged relative to the latency of the unaffected eye. During the 2nd attack, the latency of the unaffected eyes remained the same as in the 1st attack, while the latency of the affected eyes became even more prolonged (Table 6). By comparing the latency between the 1st and 2nd optic neuritis attacks, we have come to notice that there is no statistically significant spread in the latencies of the unaffected eyes, while the latency of the affected eyes increased by 9.9 ms (Table 7).
In this research, we analyzed data pertaining to subjects diagnosed with Optic Neuritis (ON). Our results have shown that during the first and second ON attacks, there was a significant decrease in visual acuity, a decrease in amplitude, and a prolongation of the P100 wave latency in the affected eye relative to the healthy eye. Also, the deterioration of all parameters was greater in the second attack than in the first.
Visual evoked potential testing was our method of choice for optic neuritis. This was based on a research by Nebbiosom [6]. which compared it with MFVEPs and FDT perimetry. The research was performed on 24 patients with optic neuritis. FDT perimetry demonstrated a decreased visual sensitivity in 12 eyes, MFVEPs in 17 eyes, and traditional VEPs in 20 eyes. Therefore, VEP tests have proven to be the most sensitive and practical diagnostic method in patients with optic neuritis. Usually MFVEPs and FDT perimetry can be used to evaluate and monitor visual impairment in patients with ON. Also, MFVEPs can be used in cases with dubious or negative findings of traditional VEPs [6].
Suppiej [7]. studied the role of visual evoked potentials in the differential diagnosis of functional visual loss and optic neuritis. VEPs have proven to be useful in the differential diagnosis of functional visual loss and optic neuritis. The results of the research have shown that out of 61 children, 49 had functional visual loss, while the remaining 12 had ON. Visual evoked potentials were normal in all patients with functional visual loss, while they were abnormal in the group with optic neuritis [7].
Our results are corroborated by a research by Chatzirallia [8]. which dealt with the measurement of retinal nerve fiber layer thickness and visual evoked potentials in MS-associated optic neuritis. 46 eyes were analyzed in 23 patients with MS who were diagnosed with unilateral or bilateral ON. Visual acuity was examined, while OCT and VEP measurements were made during the ON episode, as well as one, three and six months after the attack. The results of this research have found that, in addition to a progressive reduction in the retinal nerve fiber layer, there was a significant reduction in the amplitude and latency of the P100 wave (p<0.0001). The amplitude and latency of the P100 wave returned to normal ranges over time [8].
A research conducted by Jones S.J examined the connection between visual evoked potential parameters and acute ON, as well as ON in demyelinating diseases. The results of the research suggest that the deterioration of VEP parameters is greater in ON combined with demyelinating diseases than in acute ON, which coincides with our results. Deterioration of VEP parameters has been shown to be reversible over time. The research monitored the effect of the time interval, age, and disease stage. Visual evoked potentials were compared in six groups of patients at different time intervals after an episode of acute ON. The incidence of VEP parameter abnormalities was above 90% in patients tested during the first 6 months, but it dropped to 70% after two years. The prolongation of latency was almost 50% lower in patients tested after 2-19 years compared to the values during the acute phase (1-8 weeks). The results confirm the evidence that the shortening of latency lasts up to two years, but may last longer in younger patients. It has also been shown that electrophysiological deficiency may initially be more severe in patients with MS, but recovery might be faster [9].
Recovery of visual evoked potentials is also evident in the research that compared the interconnection of Optical Coherence Tomography (OCT) and Multifocal Visual Evoked Potentials (MFVEPs). The research was conducted by KIistorner [10]. on 25 subjects with acute unilateral ON. The subjects were analyzed over a period of 6-12 months after the attack as papilledema was expected to be in remission during that time. OCT showed a progressive axonal loss, while MFVEPs showed amplitude reduction. Compared with the results measured within 6 months, the amplitude of MFVEPs in the eye affected by ON was improved by 17.8%, while the thickness of RNFL was reduced by 20.8%. Both parameters improved after some time. The result remained unchanged regardless of the degree of optic nerve remyelination. This has confirmed that neural plasticity contributes to functional recovery after acute ON [10].
The possibility of using VEPs as biomarkers in MS and concomitant optic neuritis have been studied by Leocani [11]. VEPs play a role in assessing the extent of demyelination of the optic nerve. In addition, VEP tests can be used to predict the extent of recovery after ON and to capture the effects of clinical and subclinical demyelinating events in the afferent visual pathway [11].
Visual evoked potentials have proven to be more sensitive than Optical Coherence Tomography (OCT). Naismith [12]. have demonstrated this in a research involving 65 subjects with at least one clinical episode of optic neuritis within 6 months following the attack. Measures included clinical features, visual acuity, contrast sensitivity, OCT and VEPs. 96 clinically affected optic nerves were processed. The sensitivity of OCT to ON was 60%, while the sensitivity of VEPs was 81%. Subclinical ON of the unaltered eye was present in 32% of cases. VEPs identified 75% of all subclinically affected eyes, while OCT identified <20%. Thus, Visual Evoked Potentials (VEPs) are still preferred when it comes to detecting clinical and subclinical optic neuritis. OCT measures were not associated with disabilities and demographic characteristics that predict a poorer prognosis of MS. OCT can provide complementary information to VEPs in selected cases and remains a valuable research tool for studying optic nerve diseases across populations [12].
A comparative research of visual evoked potentials in optic neuritis and optic neuritis combined with MS was conducted by Samsen [13]. The objective of the research was to compare VEPs in patients with acute optic neuritis, recurrent optic neuritis, and ON within MS. The research analyzed 22 patients with acute optic neuritis, 8 patients with recurrent neuritis, and 22 patients with ON as part of MS. The mean value of latency of “Flash” VEPs (FVEPs) and Pattern Reversal VEPs (PRVEPs) in the group with acute ON was lower than in the group with recurrent optic neuritis. The mean latency of PRVEPs in the group with acute ON was lower than in the group with ON associated with MS. The mean value of the latency of both FVEPs and PRVEPs in the group with recurrent ON and ON associated with MS was higher, but without any statistical significance. VEPs can be used to demonstrate the demyelinating mechanisms of optic neuritis and optic neuritis within MS, but they cannot determine the susceptibility of patients with acute ON to transition to MS. Significantly delayed VEP latency in recurrent optic neuritis may be caused by a severe impairment of the optic nerve conduction due to recurrent attacks [13].
Frederiksen and Petrera performed serial measurements of VEPs in 90 untreated patients with acute optic neuritis. The patients’ age ranged from 12 to 57. VEP measurements were performed at the onset of ON and after 2, 4, 12, and 52 weeks. ON was monosymptomatic in 58 patients and part of clinically defined multiple sclerosis in 32 patients. VEPs were pathological in the eyes with acute ON in 80 out of 90 patients in one or more measurements. In the eyes with acute ON, normalization of abnormal VEPs was observed during the one-year monitoring in 13 out of 69 patients. Using a parametric analysis of variance, latency, and amplitude, combined VEP results in the eyes with acute ON have shown significant association with the time after the onset.
Latencies were significantly affected by the presence of clinically proven MS, while the amplitudes were influenced by visual acuity. The mean value of latency of VEPs in the eyes with acute monosymptomatic ON was significantly lower compared to the latency of ON that was part of clinically proven MS. The research provided evidence that VEP abnormality is often transient, as well as those VEPs often normalize after monitoring [14].
Fil ‘Chikova. conducted a research in which they studied visual evoked potentials with a pattern reversal stimulus in children with optic neuritis. VEPs were examined in 17 children with optic neuritis during the acute phase and during the recovery phase, namely 1-2 months and 1-3 years after the onset of the disease. Rough VEP changes were detected in patients during the acute phase of optic neuritis. A positive time course of the VEPs was monitored at the end of the period following an acute attack. Despite the normalization of visual acuity, there was no complete restoration of VEPs in the checkerboard pattern. The results prove that VEP research with checkerboard pattern reversal stimuli should be conducted to detect subclinical optic nerve involvement and allow for a timely diagnosis of optic neuritis in children.
Research results have shown that the visual acuity of the affected eye is significantly lower compared to the healthy eye during the first optic neuritis attack. Also, the amplitude values in the first optic neuritis attack of the affected eye are significantly lower compared to those of the healthy eye. The latency value of the P100 wave is significantly prolonged in the affected eye compared to the healthy eye in the first attack. The visual acuity in recurrent optic neuritis attacks is significantly reduced in the affected eye compared to the healthy eye.
The decrease in visual acuity of the affected eye is significantly greater in the second attack than in the first attack. Amplitude values in the recurrent optic neuritis attack of the affected eye are significantly lower compared to those of the healthy eye. The decrease in the amplitude of the affected eye is significantly greater in the second attack than in the first. The latency value of the P100 wave is significantly prolonged in the affected eye compared to the healthy eye in a recurrent attack. The prolongation of the latency of the P100 wave of the affected eye is significantly greater in the second attack than in the first.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: James L, Elice C, Victor J (2022) The Differences between Visual Evoked Potential Parameter Values in Recurrent Optic Neuritis in Patients with Demyelinating Diseases. J Clin Exp Ophthalmol. 13:918.
Received: 07-Apr-2022, Manuscript No. JCEO-22-15650; Editor assigned: 11-Apr-2022, Pre QC No. JCEO-22-15650 (PQ); Reviewed: 25-Apr-2022, QC No. JCEO-22-15650; Revised: 02-May-2022, Manuscript No. JCEO-22-15650 (R); Published: 09-May-2022 , DOI: 10.35248/2155-9570.22.13.918
Copyright: © 2022 James L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.