Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Research Article - (2023)Volume 19, Issue 1
Objective: To assess the morbidity, mortality and prognostic factors of patients with Systemic Sclerosis (SSc) and Interstitial Lung Disease (ILD).
Methods: A retrospective analysis of prospectively collected data of SSc patients between the years 2000-2020. Data collection included demographic and clinical parameters, with repeated assessments of Forced Vital Capacity (FVC), Diffusing Capacity of the Lungs for Carbon Monoxide (DLCO), and Pulmonary Artery Pressure (PAP) in SSc-ILD patients.
Results: Among 446 SSc patients [367 (82.2%) female, mean age 46.5 years, 175 (39.2%) diffuse SSc [DcSSc]], 141 (31.6%) had SSc-ILD, 121 (27.1%) deceased, of which 74 (61.6%) patients died from SSc-related complication. SSc-ILD was associated with male sex, Arab descent, DcSSc, anti-topoisomerase antibodies, myopathy, and Pulmonary Hypertension (PAH). Mortality in SSc-ILD was associated with older age, Arab descent, and PAH. Elevated PAP estimated by echocardiography at the time of SSc-ILD diagnosis, and rapid decline of FVC or DLCO, correlated with mortality. Five-year survival rates in SSc and SSc-ILD patients were constant.
Conclusion: Arab descent is an important prognostic factor for ILD and mortality. In SSc-ILD, early elevation of PAP and rapid decline in FVC or DLCO indicated a poor prognosis. SSc- related mortality is still high, and has remained stable in recent decades.
Systemic sclerosis; Interstitial lung disease; Prognostic factors; Pulmonary function tests; Survival rates
Systemic Sclerosis (SSc) is a relatively rare chronic disease of unknown etiology, characterized by disturbance in the microvasculature, dysregulation of the immune system and fibrosis. The disease can involve various organs including the heart, gastrointestinal system, skin, and more [1]. Among SSc complications, lung involvement presented by interstitial lung disease (ILD, SSc-ILD) and/or Pulmonary Arterial Hypertension (PAH) are the leading causes of SSc-related morbidity and mortality [2,3]. Pulmonary Function Tests (PFT) with comparable reduction of forced vital capacity (FVC, % of predicted) and Diffusing Capacity of the Lungs for Carbon Monoxide (DLCO, % of predicted) present a restrictive pattern of lung disease and provide a reliable tool for serial assessment during follow-up [4]. Prediction of the progressive course of lung involvement is difficult due to the heterogenicity of the disease. Different risk factors for significant SSc- ILD were identified in various studies, including males, older age, diffuse subset of SSc (DcSSc) and Anti-topoisomerase (ATA/anti Scl-70) antibodies. Reduced FVC and widespread lung involvement on high- resolution CT (HRCT) at baseline are associated with a worse prognosis [5-8,9]. Dynamic changes in HRCT and PFT reflect SSc-ILD course and correlate with poor prognosis; a higher decline rate of FVC and/ or DLCO during follow-up is predictive for adverse outcomes, including the need for oxygen supplementation and mortality [10-12], suggesting PFT decline as a surrogate prognostic factor [9].
It had been suggested that early SSc-ILD diagnosis, active approach, regular follow-up, and prompt treatment may halt the progression of pulmonary fibrosis and improve SSc-ILD outcome [4,13]. However, despite several possible treatments for SSc- ILD that demonstrated effectiveness in randomized controlled trials and have become a standard of care, among them Cyclophosphamide (CYC) [14], Mycophenolate Mofetil (MMF) [15], nintedanib [16,17], and recently tocilizumab [18-20], no significant decrease in SSc mortality was demonstrated over the last few decades and the mortality rate in SSc is still high [21], with about 35%-47.8% of mortality cases are SSc-ILD related [18,22,23], and an estimated ten-year survival rate of around 70% [8,24].
The aim of this study was to evaluate the risk factors for SSc- ILD and mortality in a large, relatively racial-homogenous cohort of Israeli SSc patients from our tertiary center, using the welldocumented electronic assessment reports from a long-term follow-up of several decades.
Study population-Adult SSc patients fulfilled SSc diagnosis according to the ACR/EULAR Classification Criteria 2013 [1], that were recruited in the years 2000-2020 at our Rheumatology Institute at Rambam Health Care Campus.
Our center is affiliated with European Scleroderma Trials and Research group (EUSTAR) registry project since 2004. Patients were recruited at one of their early visits to the clinic and followed up, according to accepted EUSTAR Minimal Essential Data (MEDs)-online.
Data collection included demographic parameters (age, sex, descent- in accordance with the patients’ national ID) and general clinical data (date of the first non-Raynaud’s phenomenon presentation [SSc diagnosis], date of the first abnormal HRCT [SSc-ILD diagnosis], disease duration [until death or the end of follow-up], SSc subset (DcSSc or Limited SSc [LcSSc], SSc clinical manifestations (Digital Ulcers [DU], pulmonary, cardiac, renal, muscular, and articular involvement), and treatment used (Methotrexate [MTX], Azathioprine [AZA], MMF, CYC, biologic treatment including rituximab and tocilizumb, intedanib]). Laboratory data included Autoantibodies status (ATA, Anticentromere [ACA], Anti RNA Polymerase 3 [RNAP3] antibodies), PFT [FVC, DLCO]); Imaging (X-rays or HRCT or Chest CT), and ECHO cardiography (Ejection Fraction, % [EF], Pulmonary Artery Pressure [PAP, mm Hg]).
All SSc patients underwent baseline lung evaluation including lung imaging, PFT and ECHO. The values of PAP, FVC and DLCO recorded during the period from one month before SSc- ILD diagnosis until 6 months following SSc-ILD diagnosis, were defined as “close to ILD diagnosis”.
Patients with early SSc (5-7 years from the first non-Raynaud’s phenomenon), DcSSc, positive ATA and those with respiratory symptoms, abnormal HRCT and/or PFT underwent repeated chest HRCT, regular PFT and ECHO evaluations at 6-12 months intervals. Patients with clinically significant SSc-ILD were referred to and followed up in the multidisciplinary combined rheumatology-pulmonology (fibro-vascular) clinic every 3-6 months.
Patients’ data was collected in Excel files with an annual checklist of assessment; missing data on registered patients was extracted via “Prometheus” software medical records for the period between January 2000 and September 2020. The study was approved by the Institutional Review Board (protocol # RMB 354-20).
Definitions
SSc-ILD was defined according to findings on chest CT/HRCT (ground glass opacities, reticular changes, traction bronchiectasis, honeycombing). In each patient, imaging tests were reviewed by both radiology and rheumatology specialists.
FVC or DLCO predicted values lower than 80% were defined as reduced. Pulmonary hypertension was defined as an estimated PAP above 40 mmHg on ECHO. Heart involvement was defined as the presence of pericardial effusion, Ejection Fraction (EF) below 50%, significant diastolic dysfunction, Congestive Heart Failure (CHF), arrhythmia requiring treatment, and complete atrial-ventricular block. Esophageal reflux, esophageal dilatation on chest CT, gastric antrum vascular ectasia, symptoms of small intestinal bacterial overgrowth or intestinal emergency comprised Gastrointestinal Tract (GIT) involvement. DU, gangrene, critical digital or toe ischemia were reported as vascular complications. Scleroderma Renal Crisis (SRC) and renal failure were reported as renal involvement. SSc-related myopathy was considered in cases of creatine kinase elevation (CK, × 1.5 time of upper limit), muscle weakness and/or wasting, sarcopenia, positive muscle magnetic resonance imaging or positive muscle biopsy. Arthritis, tendon friction rubs, sclerodactyly and joint contractures were attributed to articular involvement.
Dynamic changes in FVC and DLCO were assessed in patients with multiple PFT during follow-up. We defined patients with “rapid dynamics” if they had an average decrease in FVC or DLCO per month greater than the patients’ median value (faster decline than half of the patients) and patients with “slow dynamics” with an average monthly decrease in FVC or DLCO lower than the patients’ median value (slower decline than half of the patients).
Statistical analysis
Demographic and clinical variables were compared between SSc- ILD and SSc-non ILD, using Chi-Squared test or Fisher exact test for categorical variables; T-test was used for continuous variables. Total survival and five-year survival rates between groups were compared using Kaplan-Meier curves, and the difference in survival was tested using a log-rank test. Predictors for survival were assessed using backwards selection procedure Cox proportional hazard regression models with the stepwise model selection procedure. The comparison of mean test values between patients who died and survived during the follow-up period was assessed using the Cox regression analysis. A comparison of patients’ survival according to FVC values measured close to the time of SSc-ILD diagnosis, was performed using Kaplan Meyer curves, and the difference in survival rates was tested using log-rank test. Univariate cox regression was used to study the association between continuous variables (PAP, FVC and DLCO values) and mortality during the follow-up period. P-value<0.05 was accepted as significant.
We collected data on 470 SSc patients; 24 patients were lost to follow-up or had insufficient data to define pulmonary involvement, and therefore were not included in the analysis. Among the rest of the 446 SSc patients included in the analysis: 367 (82.2%) were females; the mean age at SSc diagnosis was 46.5 ± 11.6 years; 175 (39.2%) patients had DcSSc; 141 (31.6%) patients were diagnosed with SSc-ILD.
The incidence of SSc-ILD was significantly higher in the Arab population. Among SSc-ILD patients, DcSSc, ATA autoantibodies, myopathy and PAH were significantly more prevalent. SSc-ILD patients were treated more with AZA, CYC, MMF and nintedanib; numerically more SSc-ILD patients with a trend to significance were treated with biological agents as shown in Table 1.
| Parameters | SSc-non ILD n=305 |
SSc-ILD n=141 |
P value |
|---|---|---|---|
| Male (n,%) | 50 (16.4%) | 29 (20.6%) | 0.428 |
| Arab descent (n,%) | 57 (18.7%) | 48 (34%) | 0.002 |
| Mean age at diagnosis (years) ± SD | 46.5 ± 14.4 | 47.6 ± 14.2 | 0.528 |
| Mean duration of disease (years)±SD | 11.6 ± 8.7 | 10.1 ± 7.6 | 0.105 |
| Death cases (n,%) | 75 (24.6%) | 46 (32.6%) | 0.145 |
| Death, related to SSc (n,%) |
38 (50.7%) | 36 (78.3%) | 0.016 |
| Mean age at death (years) ± SD | 67 ± 13.7 | 63.7 ± 13.7 | 0.205 |
| Diffuse SSc subtype (n,%) |
78 (25.6%) | 97 (68.8%) | 0.00001 |
| Antibodies ATA (%) ACA (%) |
80 (26.2%) 125 (41%) |
91 (64.5%) 15 (10.6%) |
0.002 0.002 |
| Myositis (n,%) | 31 (10.2%) | 30 (21.3%) | 0.007 |
| Pulmonary arterial hypertension (n,%) | 68 (22.3%) | 49 (34.8%) | 0.018 |
| Scleroderma renal crisis (n,%) | 11 (3.6%) | 7 (5.0%) | 0.675 |
| Heart involvement (n,%) | 53 (17.4%) | 36 (25.5%) | 0.105 |
| Treatment (n,%)- [ever] Methotrexate Azathioprine Mycophenolate mofetil Cyclophosphamide Biologic treatment* Nintedanib IVIG |
111 (36.4%) 35 (11.5%) 36 (11.8%) 19 (6.2%) 43 (14.1%) 0 (0%) 24 (7.9%) |
47 (33.3%) 52 (36.9%) 72 (51.1%) 64 (45.4%) 31 (22.0%) 4 (2.8%) 18 (12.8%) |
0.665 0.00001 0.00001 0.00001 0.098 0.020 0.197 |
Abbreviations: SSc – Systemic Sclerosis, ILD – Interstitial Lung Disease, ATA - Anti Topoisomerase Antibodies, ACA – Anti Centromere Antibodies, SD – Standard Deviation, IVIG – Intravenous Immunoglobulins.
Note: * Biologic treatments: rituximab, abatacept, tocilizumab, etanercept
Table 1: Patients characteristics of SSc-non ILD and SSc-ILD patients.
During the follow-up period, 121 (27.1%) patients died; 74 (61.1%) deaths were related to SSc and its complications: 25.6% died from respiratory failure (ILD or PAH or both); 7.4%, 21.4%, 6.6% and 4.1% died from cardiac complications, infections, GIT complications, and renal involvement, respectively. Significantly more SSc-related death was reported in patients with SSc-ILD (36 [78.3%] vs 38 [50.7%] in SSc non-ILD, p<0.016). Survival was significantly reduced in SSc-ILD patients compared to the general SSc group and SSc-non-ILD patients as shown in Figure 1, p<0.01.
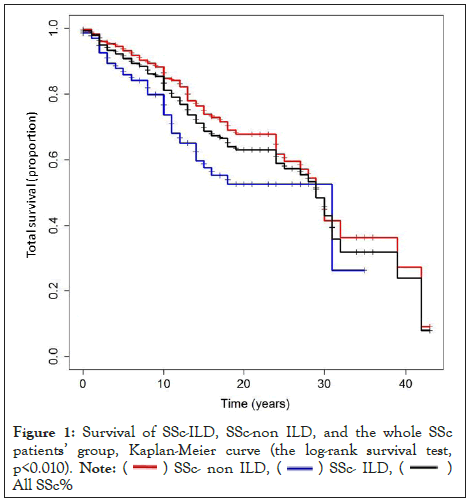
Figure 1: Survival of SSc-ILD, SSc-non ILD, and the whole SSc
patients’ group, Kaplan-Meier curve (the log-rank survival test,
p<0.010).
Note: ( ) SSc- non ILD, (
) SSc- non ILD, ( ) SSc- ILD, (
) SSc- ILD, ( )
All SSc%
)
All SSc%
In the whole SSc cohort, mortality was strongly associated with male gender (Hazard Ratio [HR] 1.78, p<0.01); Arab descent (HR 2.03, p<0.01); age at diagnosis (HR 1.09, p<0.01); DcSSc (HR 1.76, p<0.01); myopathy (HR 2.25, p<0.01); cardiac involvement (HR 1.99, p<0.01); cyclophosphamide treatment (HR 1.98, p<0.01).
In the SSc-ILD subgroup, a similar strong association was found between mortality and Arab descent (HR 3.53, p<0.01), older age at diagnosis (HR 1.09, p<0.01), PAH (HR 3.09, p<0.01), and cyclophosphamide treatment (HR 2.81, p<0.01). Cumulative survival rates in the general SSc cohort were 99.3%, 93.4%, 90.8%, and 81.2% at 1,3,5 and 10 years, respectively. Cumulative survival rates in SSc-ILD patients were 97.8%, 94.4%, 87.9% and 72.2% at 1,3, 5 and 10 years, respectively.
We compared five-year survival rates of all SSc patients and SSc-ILD patients, in different periods, divided by the years of diagnosis: 2001-2005, 2006-2010, 2011-2015, using Kaplan– Meier curve (data is not shown). Five- year survival in the general SSc group was 91.8%, 91.2% and 87.3% respectively, and 85.7%, 89.7% and 81.6% respectively in SSc-ILD patients. There were no significant changes in five-year survival rates between different periods of diagnosis, in the whole SSc group, and in SSc-ILD patients.
SSc-ILD patients
Among 141 SSc-ILD patients, 104 patients had available data on estimated PAP on ECHO; 102 patients had available data on initial and repeated FVC, and 99 had available data on DLCO.
Median (interquartile range, IQR) values recorded at the first year after ILD diagnosis were PAP 35 mmHg (n=55, 30-38 mmHg), FVC 75% (n=37, 58%-88%) and DLCO 62% (n=37, 49%-73%). We compared PAP, FVC and DLCO values obtained at the first year after ILD diagnosis, between patients who had died during follow-up and survived patients (Table 2). Among these values, only elevated PAP at the time of ILD diagnosis was significantly associated with poor survival. Analyzes of survival according to FVC values at the time of ILD diagnosis using the Kaplan- Mayer curve did not demonstrate a significant difference between 23 patients with FVC>70% and 14 patients with FVC<70% (p=0.323). The same results were obtained with FVC limits below and above 80% and 65% of predicted (data is not shown).
| Values | Deceased patients | Survived patients | P value | HR | 95 % CI for HR |
|---|---|---|---|---|---|
| PAP mm Hg (mean, SD) |
38.24 (12.28) | 34.29 (8.51) | 0.002 | 1.055 | 1.019-1.092 |
| FVC % (mean, SD) |
69.69 (21.69) | 71.97 (16.02) | 0.398 | 0.985 | 0.951-1.020 |
| DLCO % (mean, SD) |
53.86 (14.86) | 63.64 (16.55) | 0.069 | 0.962 | 0.923-1.003 |
Abbreviations: SSc - Systemic Sclerosis, ILD - Interstitial Lung Disease, PAP - Pulmonary Artery Pressure, FVC - Forced Vital Capacity, DLCO - Diffusion Capacity of the Lung for Carbon monoxide, HR - Hazard Ratio, CI - Confidential Interval, SD - Standard Deviation
Table 2: Prognostic value of baseline PAP, FVC and DLCO in SSc- ILD
”Rapid” vs “Slow” PFT dynamics analysis
According to our collected data, each patient has a different amount of PFT results (FVC, DLCO), with various time differences between tests. To create uniformity, a variable of the time elapsed between the diagnosis and each test, and a variable of the mean difference in PFT values (FVC, DLCO) over one month was created.
Patients with sufficient data were then divided into “rapid dynamics” with an average decrease in FVC or DLCO per month greater than the patients’ median value (faster decline than half of the patients) and “slow dynamics” with an average monthly decrease in FVC or DLCO lower than the patients’ median value (slower decline than half of the patients). The “rapid dynamics” FVC monthly reduction, with an average follow-up of 44 months, was -0.78% on average. The “slow dynamics” FVC reduction average follow-up was 63 months, with an average change of +0.23% per month. The “rapid dynamics” DLCO monthly reduction, with an average follow-up 57 months, was -0.83%. “Slow dynamics” DLCO change (average follow-up 66 months) was +0.11%. The average decline of FVC per month was-0.01% in surviving patients, compared to -0.25% in patients who died during the follow-up period (p=0.018). The average decline of DLCO per month was -0.16% in patients who survived, compared to -0.93% in patients who died during the follow-up period (p<0.001). The mortality rate in the group of “rapid dynamics” FVC was 43.5%, compared to 16.9% in “slow dynamics” FVC (p=0.012). The mortality rate in the group of “rapid dynamics” DLCO was 38.1% compared to 14.6% in “slow dynamics” DLCO (p=0.015). Kaplan-Mayer curve on survival of patients with “rapid dynamics” and “slow dynamic” of FVC and DLCO showed a significant difference between these subgroups of SSc-ILD patients as shown in Figure 2 and Figure 3, the logrank survival test, p<0.001 and 0.015, respectively).
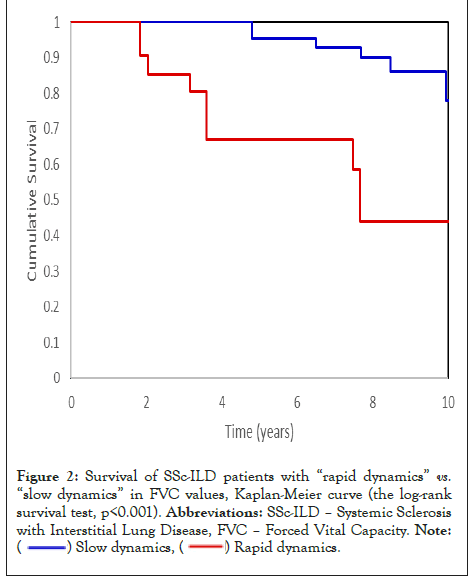
Figure 2: Survival of SSc-ILD patients with “rapid dynamics” vs. “slow dynamics” in FVC values, Kaplan-Meier curve (the log-rank
survival test, p<0.001). Abbreviations: SSc-ILD – Systemic Sclerosis
with Interstitial Lung Disease, FVC – Forced Vital Capacity.
Note: ( ) Slow dynamics, (
) Slow dynamics, ( ) Rapid dynamics.
) Rapid dynamics.
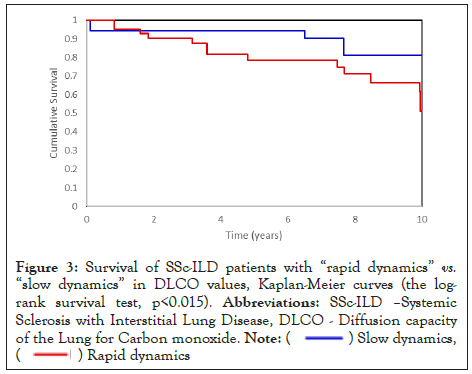
Figure 3: Survival of SSc-ILD patients with “rapid dynamics” vs. “slow dynamics” in DLCO values, Kaplan-Meier curves (the logrank
survival test, p<0.015). Abbreviations: SSc-ILD –Systemic
Sclerosis with Interstitial Lung Disease, DLCO - Diffusion capacity
of the Lung for Carbon monoxide.
Note: ( ) Slow dynamics,
(
) Slow dynamics,
( ) Rapid dynamics
) Rapid dynamics
We analyzed a large and ethnically homogeneous cohort of SSc patients in a tertiary center with a well-documented prospective follow-up. Patients in our cohort demonstrated similar demographics and clinical parameters as described in the literature, with a prevalence of SSc-ILD of around one-third of patients [25]. Distler et al. demonstrated a prominent discrepancy between robust female predominance in SSc and strong male predominance among SSc-ILD with a relative risk of 1.24 to develop ILD [13]; in our cohort, we observed numerically more males in SSc-ILD cohort, but the difference was not significant.
As in other cohorts, our patients with SSc-ILD had a high prevalence of DcSSc, ATA antibodies, myopathy, and PAH; the incidence of SSc-ILD was low in patients with ACA antibodies (10.6%). Patients with SSc-ILD were more often treated with immunosuppressive drugs such as MTX, AZA, MMF, and CYC [14-17]. High rates of mortality in patients treated with CYC may be explained by the fact that patients with more severe and progressive SSc and SSc-ILD were treated with CYC, and less likely due to drug toxicity. Nintedanib was not available in Israel for patients with SSc-ILD until the year 2020; only four patients had received this treatment at the time of analysis.
A comparison between SSc-ILD and SSc-non-ILD patients showed a significantly higher prevalence of ILD in Arab descent. A higher prevalence of SSc, and specifically SSc-ILD, was previously reported among African American patients [26,27]; no comparison between Jews and Arabs was reported in the literature. These findings can be explained by several factors; the frequency of industry exposure to silica in Arab male patients who are commonly working in construction and stone industries; genetic factors, as consanguineous marriages are still prevalent in the traditional Arab population in Israel, and lower socioeconomic status with residence in rural and peripheral areas with lower access to medical services. This finding strengthens the hypothesis that a combination of genetic and environmental factors are involved in SSc, and particularly in SSc-ILD.
During the follow-up period, 121 (27.1%) patients died; 74 (61.1%) deaths were related to SSc and its complications. Survival in our cohort was dependent on lung involvement and was significantly lower in SSc-ILD compared to the general and particularly to SScnon- ILD group. Our findings are similar to the data described in several patient cohorts: the EUSTAR database 55% [28], the Spanish cohort 55% [29], the Turkish cohort 66.6% [30] but higher than in a single center British cohort 35.8% [31], Italian cohort 27.6% [32], and 26.0% in Danish cohort [33]. In a metaanalysis of studies reporting death cases in SSc patients, Rubio- Rivas et al [22] analyzed data from 40 studies with 13,679 cases; total mortality was 22.7%, with SSc-related death in 47.6% of patients, mainly from lung involvement.
Organ involvement is reported as one of the main SSc-related complications associated with a higher mortality rate [8, 34-36]. Steen et al. reported that the nine-year cumulative survival rate of SSc patients with significant single internal organ involvement demonstrated half of the survival rate in patients without internal organ complications [37]. In our cohort, DcSSc, myopathy and heart involvement were associated with mortality in the general SSc group; elevated PAP on ECHO was the main component associated with SSc-ILD-related mortality. The strong association between elevated PAP and mortality most likely reflects the complexity of lung and heart involvement in SSc; elevated PAP may be a result of progressive ILD, primary PAH, or CHF, which are frequently reported in SSc patients.
Treatment with CYC followed by AZA or MMF was the main treatment regimen in the years 1995-2010. It is noteworthy that our data reflect a cohort of more severe SSc patients, which explains the higher rates of CYC treatment. The high hazard ratio for CYC in the general SSc and particularly SSc-ILD group, mainly reflects the treatment approach for more severe patients, than an association with mortality.
Over the last ten years, biologic agents (rituximab, abatacept, tocilizumab) were used more often in patients with progressive skin and lung disease. Because of the low number of patients treated with biologics and/or nintedanib and a short period of use, we could not draw conclusions on this topic. The growing trend for use of biologic agents in patients with SSc-ILD was reported in the EUSTAR cohort [38].
An accelerated decline rate of FVC and elevation of PAP are important prognostic factors for mortality as demonstrated in our cohort, and in compliance with previous data [39-43]. Serial assessments of FVC and DLCO seem to be necessary for patient evaluation, in order to provide a better patient selection for intensive anti-inflammatory and anti-fibrotic therapy, thereby improving patients’ prognosis and outcome.
Five-year survival rates between groups of patients, divided by the year of SSc diagnosis (2001-2005, 2006-2010, and 2011-2015); were 91.8%, 91.2% and 87.3% respectively. These mortality rates are in accordance with the described in the literature [29,44] and did not change significantly during the study period.
Studies that examined SSc survival trends over the last decades, showed inconsistent results [21,28,45]; the majority of them were observational and retrospective with possible missing data biases. For example, Elhai et al. demonstrated that in SSc patients (2719 French cases and 1072 cases from EUSTAR cohort) the standardized mortality ratio did not significantly change over the past four decades [21]. Despite great progress in the understanding of SSc and SSc-ILD pathogenesis, as well as a generally more proactive organs assessments and problem-focused approach to SSc, survival is still poor, especially in SSc [22].
According to Su et al [46], survival rates of our SSc-ILD patients were lower than in our general SSc group during each treatment period; nonetheless, they were similar to previously published data without significant difference between the five-year time intervals. Examining the majority of survival curves in past data, it seemed that in SSc, and SSc-ILD in particular, five-year survival may not be a sufficient measure to reflect SSc patient progression; in the majority of published data, the differences in survival rates begin at year 7, 10 and even 12 after SSc diagnosis. A numerical non-significant reduction in survival rate during the years 2011-2015 may be explained by a relatively large number of severe patient cohort with DcSSc and SSc-ILD, relative to previously reported cohorts. Another possible explanation for this finding might be the fact that we did not include the years 2016-2020 in our analysis, when other treatment options for SSc and ILD became more available and diverse, as we limited the data collection to September 2020. One of our thoughts to limit the analysis by year 2020 was the pandemic as we could expect elevated mortality in our patients related to Covid-19 in the first place.
Among the limitations of the study, are a relatively small number of patients with multiple PFT values with different time intervals between repeated assessments, and a retrospective method of data analysis.
The strengths of our study include a large cohort of patients from the same tertiary center with a multidisciplinary team, which included close monitoring and follow-up and documented in a computerized database. In addition, a didactic diagnosis of ILD, based on the existing definitions and careful patient evaluation by dedicated specialists team. In the vast majority of patients, we had precise dates of SSc and SSc-ILD diagnosis based on chest CT/HRCT and PFT, regular PFT and ECHO data, accurate registration of mortality and death cause, as well as an accurate registration of treatment.
SSc-ILD has a major negative impact on patients’ outcomes, and serial assesment with PFT and ECHO are imperative for patient assessment and treatment optimization. Arab descent is an important prognostic factor in SSc patients, including SSc-ILD. Survival of SSc-ILD patients remains reduced; there is a hope that recently developed anti-fibrotic drugs and biologic agents will improve future outcomes in patients with SSc and SSc-ILD.
There were no financial support or other benefits from commercial sources to declare regarding this article; each author confirmed that there were no financial interests or conflicts of interests concerning the study.
Citation: Keret S, Markovits D, Giryes S, Tavor Y, Dolnikov K, Gurman AB, et al (2023) Systemic Sclerosis- Interstitial Lung Disease Characteristics and Survival Rates in a Long-term Follow-up. Immunome Res. 19: 226
Received: 01-Feb-2023, Manuscript No. IMR-23-21665; Editor assigned: 06-Feb-2023, Pre QC No. IMR-23-21665 (PQ); Reviewed: 20-Feb-2023, QC No. IMR-23-21665; Revised: 27-Feb-2023, Manuscript No. IMR-23-21665 (R); Published: 06-Mar-2023 , DOI: 10.35248/1745-7580.23.19.226
Copyright: © 2023 Keret S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.