Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2018) Volume 9, Issue 2
In this current investigation functionalization of psyllium has been done with acrylic acid in the presence of potassium per-sulphate (KPS)-hexamethylene tetramine (HMTA) as an initiator-crosslinker system. After the initial optimization of different parameters for the synthesis of the hydrogel, the candidate polymer, i.e., (Psy-cl-poly(AA)- IA, has been studied for its sustained release behavior of ‘benomyl’ fungicide for its further use in agricultural sector. The diffusion exponent ‘n’ for Psy-cl-poly(AA)-IA has been found to be 1.16. The ‘n’ value indicated Case-II type diffusion.
Keywords: Biopolymer; Psyllium; Acrylic acid; Sustained fungicide release
Recent years have witnessed tremendous applications of pesticides, herbicides, fungicides and fertilizers on agricultural land. These chemicals were exploited without considering the disadvantages of the same. After being used for at-least two to three decades, the world is now observing the hazardous effects of these chemicals. The properties of pesticide such as volatility, leaching and photo-degradation which led to increased dosage of pesticides. Excess pesticides in the soil can be harmful to us in many ways. Out of the total amount of applied pesticides, it is only the little amount (5-10%), which is effectively used, rest being lost in many ways. These lost pesticides are one of the major components of water pollution and can harm living species by intruding the food chain. These chemicals act on the nervous system, circulatory system and genetic system, hence, creating disorders which may be carried over to generations together.
Controlled release of pesticides can thus be a major remedial factor. The release mechanism follows the processes such as diffusion, degradation or a combination of both. Controlled pesticide delivery using polymeric materials has not been much explored by the scientists worldwide. Use of polymeric matrix such as alginate [1,2], ethyl cellulose [3], lignin [4,5] and starch [6-10] for the controlled and sustained release of pesticides has been earlier reported in the literature. Dogan [11] studied the in vitro effect of pesticides such as lambda-cyhalothrin, deltametrin, diozinon, dorzolamide and brinzolamide on carbonic anhydrase enzyme obtained from the blood of fish Oncorhynchus mykiss and Cyprinus carpio and compared it with carbonic anhydrase inhibitors. Fernandez-Perez et al. [12] studied the use of polymeric matrix to reduce the leaching of herbicide (diuron) into the soil and hence the ground water contamination. The use of polymeric matrix loaded with pesticides help in attaining sustained activity [13], reducing evaporation, photo-degradation [14] and leaching [15].
The present study dealt with the controlled and sustained release of pesticide (Benomyl) using bio-polymer i.e., psyllium-based hydrogels, crosslinked with acrylic acid as a monomer and using potassium persulphate- hexamethylene tetramine as an initiator-crosslinker system.
Plantago Psyllium (Sidhpur Sat-Isabgol Factory), acrylic acid (Merck-Schuchardt), potassium per sulphate (S. D. Fine), hexamethylene tetramine (S. D. Fine) and Benomyl (IFFCO) were used as received. Electronic balance (Libror AEG-220, Shimadzu) was used for weighing purpose.
FTIR spectra of the samples were taken on Perkin Elmer RXI Spectrophotometer using KBr pellets. Scanning electronic microscopic measurements of the samples were taken on Jeol Steroscan 150 Microscope. Thermo-gravimetric Analysis/Differential Thermal Analysis (TGA/DTA) studies were carried-out on Linseis, L-81 11 thermal analyzer in air at a heating rate of 10°C/min. X-ray diffraction studies were carried-out on X-ray diffractometer (Bruker AXS D8 Advance). Crystalline index (C.I.), which measures the orientation of the crystals in a polysaccharide to the polysaccharide axis, was determined by using the wide-angle X-ray diffraction counts at 2θ angle close to 21.297° and 34.744°. The counter reading at peak intensity of 21.297° is said to represent the crystalline material and the peak intensities at 34.744° corresponds to the amorphous material in Psyllium [16]. Percentage crystallinity (%Cr) [17] and the crystalline index [18] were calculated as follows:
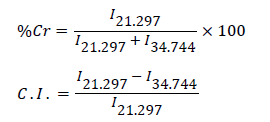
where I21.297 and I34.744 are the crystalline and amorphous intensities at 2θ scale close to 21.297° and 34.744°, respectively. The concentration of the fungicide at definite time interval was studied using UV-VIS spectrophotometer (UV-VIS Spectrophotometer- Systronics 118), the fungicide loaded polymers were dried at 40°C using thermostatic incubator (Scientific Instruments Ltd.) and the weighing was performe
Sample preparation
Synthesis of different crosslinked graft copolymers of Psyllium with acrylic acid was carried-out in-air (IA). Psyllium (1.0 g) was taken in a flask having a known amount of solvent. A known amount of monomer was added to this reaction mixture followed by the addition of an initiator and crosslinker dissolved previously in minimum amount of the solvent. Mixture was made uniform by thorough stirring. The reaction was carried-out under required temperature. After completion of reaction, the homopolymer was removed with water. The synthesized polymers were dried to constant weight in hot air oven at 50°C. Optimization was carried-out as a function of percent grafting (Pg) and percent swelling (Ps) which were calculated as per the equations [19,20]:
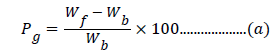
where Wf=weight of functionalized polymer, Wb=weight of backbone polymer.
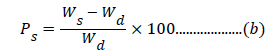
where, Ws and Wd are the weights of swollen polymer and dry polymer, respectively.
Swelling studies of candidate polymer
The purpose of swelling studies is to optimize the different conditions best suited for maximum swelling. These studies in distilled water have been carried-out using gravimetric method as a function of time (2, 4, 8, 16 and 24 h) and temperature (25, 37, 45 and 50°C). 100 mg of the polymer sample was immersed in 250 ml of water. Sample was taken-out after a definite interval of time, wiped and weighed. The process was repeated till a constant weight was reached. The percent swelling was calculated as per the following equation.
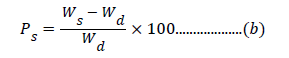
After optimization of time as a function of Ps, the temperature for maximum percent swelling was optimized.
Loading of fungicides onto polymeric matrices
Saturated aqueous solution of fungicide was prepared and λmax was noted down. 750 mg of polymeric sample was immersed in 100 ml of fungicide solution for 24 h. The polymer was then taken-out from the solution, wiped off with tissue paper and was kept for drying in incubator at 40C. Dried fungicide-loaded polymer was washed with distilled water to remove any surface adhered molecules and was studied for fungicide release kinetics as a function of release time.
Mathematical analysis: Mathematical modeling of fungicide release with hydrogel was studied as per the methods reported earlier in the literature [21-25]. The empirical equation used to describe the water uptake which is the weight gain (Ms) can be presented as [26].
Ms=Ktn ………………..(1)
where, ‘k’ and ‘n’ are constant. ‘n’=0.5 reveals the normal Fickian diffusion whereas ‘n’=1.0 signifies Case II diffusion. Non-Fickian or anomalous diffusion is characterized with value of n between 0.5 and 1.0 [26]. Evaluation of fungicide release from the swellable polymers can be assessed from the above power law expression. Here, Ms is replaced with Mt/M∞ and the expression is modified as [27]:
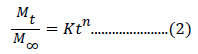
where, Mt/ M∞ is the fractional release of fungicide in time t. ‘k’ is the constant which is characteristic of polymer-fungicide system. ‘n’ is the diffusion exponent characteristic of the release mechanism. Value of ‘n’ and ‘k’ can be evaluated from the slope and intercept of the plot between ln Mt/ M∞ versus ln t, respectively. This equation can be applied until 60% of the total fungicide is released.
Diffusion coefficients: Analysis of fungicide release from various hydrogels can be performed by calculating the diffusion coefficients. Diffusion process can be adequately described through Fick’s first and second law. Integral diffusion for the cylindrical hydrogel can be given as [28]:
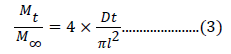
where, Mt/ M∞ is the fractional release, Mt is the fungicide released at time ‘t’, and M∞ is the fungicide release at equilibrium, D is the diffusion coefficient and l is the thickness of the sample.
The average diffusion coefficient (DA) for the 50% release of the fungicide can be calculated by putting Mt/ M∞=0.5 in Eq. 3, and can be presented as:

where, t1/2 is the time required for the 50% release of fungicide.
Eq. 5 gives the value of late diffusion coefficient and can be calculated as [27,28]:
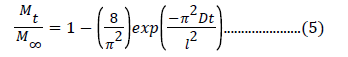
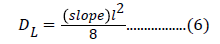
The slope of plot between ln(1- Mt/ M∞) and time ‘t’ has been used for the evaluation of DL [27].
Optimization of different reaction parameters
The different reaction parameters such as initiator concentration, amount of solvent, reaction time, reaction temperature, pH, monomer concentration and crosslinker concentration were optimized and the scheme of results for optimization of the reaction parameters is illustrated in Table 1 [29].
| S. No. | Initiator Conc. | Amount of solvent (ml) | Reaction time (min.) | Reaction temperature(⁰C) | pH | Monomer conc.mol/L | Crosslink-er conc. mol/L | Percent grafting | Percent swelling |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 0 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 0 | - |
| 2. | 9.24 × 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 73 | - |
| 3. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 125 | - |
| 4. | 27.74× 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 84 | - |
| 5. | 36.99× 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 81 | - |
| 6. | 18.49× 10-3 | 10 | 120 | 65 | 7 | 0.725 | 0.02853 | 32 | - |
| 7. | 18.49× 10-3 | 15 | 120 | 65 | 7 | 0.725 | 0.02853 | 61 | - |
| 8. | 18.49× 10-3 | 20 | 120 | 65 | 7 | 0.725 | 0.02853 | 118 | - |
| 9. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 124 | - |
| 10. | 18.49× 10-3 | 30 | 120 | 65 | 7 | 0.725 | 0.02853 | 80 | - |
| 11. | 18.49× 10-3 | 25 | 30 | 65 | 7 | 0.725 | 0.02853 | 0 | - |
| 12. | 18.49× 10-3 | 25 | 60 | 65 | 7 | 0.725 | 0.02853 | 116 | - |
| 13. | 18.49× 10-3 | 25 | 90 | 65 | 7 | 0.725 | 0.02853 | 118 | - |
| 14. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 125 | - |
| 15. | 18.49× 10-3 | 25 | 150 | 65 | 7 | 0.725 | 0.02853 | 90 | - |
| 16. | 18.49× 10-3 | 25 | 120 | 55 | 7 | 0.725 | 0.02853 | 97 | - |
| 17. | 18.49× 10-3 | 25 | 120 | 60 | 7 | 0.725 | 0.02853 | 118 | - |
| 18. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 125 | - |
| 19. | 18.49× 10-3 | 25 | 120 | 70 | 7 | 0.725 | 0.02853 | 110 | - |
| 20. | 18.49× 10-3 | 25 | 120 | 75 | 7 | 0.725 | 0.02853 | 0 | - |
| 21. | 18.49× 10-3 | 25 | 120 | 65 | 4 | 0.725 | 0.02853 | 5 | - |
| 22. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.725 | 0.02853 | 125 | - |
| 23. | 18.49× 10-3 | 25 | 120 | 65 | 9 | 0.725 | 0.02853 | 9 | - |
| 24. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.29 | 0.02853 | - | 2309 |
| 25. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.58 | 0.02853 | - | 3244 |
| 26. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.807 | 0.02853 | - | 3090 |
| 27. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 1.16 | 0.02853 | - | 2466 |
| 28. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 1.45 | 0.02853 | - | 2192 |
| 29. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.58 | 0.01426 | - | 2000 |
| 30. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.58 | 0.02853 | - | 3200 |
| 31. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.58 | 0.04279 | - | 2987 |
| 32. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.58 | 0.05706 | - | 2875 |
| 33. | 18.49× 10-3 | 25 | 120 | 65 | 7 | 0.58 | 0.07133 | - | 2600 |
Table 1: Scheme for optimization of various reaction parameters.
FTIR spectroscopy
The IR spectrum of Psyllium [29] showed broad peaks at 3780.9 cm-1 and 3427.6 cm-1 (O-H stretching bonded absorption of carbohydrates), 2925.8 cm-1(CH2 asymmetric stretching), 1378.8 cm-1(CH, CH2 and OH in-plane bending in carbohydrates), 1039.5 cm-1 (C-O stretching region as complex bands, resulting from C-O and C-O-C stretching vibrations), 897 cm-1 and 533 cm-1 (pyranose rings). Whereas, Psy-cl-poly(AA)-IA showed peaks at 2857.1 cm-1 (carboxylic acid O-H stretching), 1737.9 cm-1 (C=O stretching in carboxylic acid), 1636.4 cm-1 (strong C……O asymmetric stretching) and 1400 cm-1 (coupled OH in-plane bending and C-O stretching) besides peaks obtained with that of Psyllium.
Scanning electron microscopic studies (SEM)
SEM of psyllium and Psy-cl-poly(AA)-IA clearly indicated the morphological changes which are brought about as a result of crosslinking onto the backbone [29].
Thermogravimetric analysis and differential thermal analysis studies (TGA/DTA)
Thermo-gravimetric analysis of Psyllium and Psy-cl-poly(AA)-IA was carried-out as a function of percent weight loss versus temperature at a rate of 10°C/min in air. The analysis was performed in order to examine the changes in thermal properties of the Psyllium brought about by graft copolymerization with acrylic acid in air [29]. Psyllium exhibited initial decomposition temperature (IDT) at 229.3C and final decomposition temperature (FDT)) at 601.9°C. Two stage decomposition has been found between 229.3°C to 316.4°C (wt. loss=47.16%) and 316.4°C to 601.9°C (wt. loss=38.0%). DTA curve of Psyllium indicated one endothermic peak at 67.1°C (-2.3 μV) and two exothermic peaks at 298.9 °C (12.7 μV) and 487.9°C (16.0 μV). It has been found that in case of Psyllium, IDT (229.3°C) is higher as compared to that of Psy-cl-poly(AA)-IA (172.2°C). However, FDT of Psy-cl-poly(AA)-IA has been found to be higher (620.5°C) than that of Psyllium (601.9°C). Two-stage decomposition from 172.2°C to 496.1°C (wt. loss=40.8%) and 496.1°C to 620.5°C (wt. loss=31.0%) was obsereved. DTA studies of this crosslinked sample showed one endothermic peak at 138.7°C (-3.1 μV) and one exothermic peak at 534.3°C (22.4 μV). It shows that exothermic combustion of Psy-clpoly( AA)-IA persists at higher temperature as compared to that of Psyllium [30].
X-Ray diffraction (XRD) studies
It has been observed that Psyllium exhibited 75.76% percentage crystallinity and crystalline index was found to be 0.6800. Whereas, Psy-cl-poly(AA)-IA exhibited 64.91% percentage crystallinity and the crystalline index of 0.4592 (Table 2).
| S. No. | Sample | Pg ± SE | at 2θ-Scale | %Cr | C.I. | |
|---|---|---|---|---|---|---|
| I21.297 | I34.744 | |||||
| 1 | Psyllium | - | 100 | 32 | 75.76 | 0.68 |
| 2 | Psy-cl-poly(AA)-IA | 125.0 ± 1.00 | 98 | 53 | 64.91 | 0.4592 |
Table 2: Percentage crystallinity (%Cr) and Crystalline Index (C. I.) of Psyllium and Psy-cl-poly(AA). Where, I=relative intensity; IA=in air; Pg=percent grafting.
It is evident from Figure 1 that spectrum of Psyllium is more convex than that of Psy-cl-poly(AA)-IA. The incorporation of monomer moiety onto the Psyllium had impaired its crystallinity, thereby decreasing the %Cr [31]. The 2 θ, I and d-values of graft copolymer has been found to be quite different than that of Psyllium. Psy-clpoly( AA)-IA showed the relative intensity of 49 (d=2.2175) at 42.9° 2 θ-scale. This behaviour is due to the involvement of primary bonding like covalent bonding between Psyllium and acrylic acid during graft copolymerization [16].
Swelling studies of the candidate hydrogel
Effect of time on percent swelling: Figure 2a reveals the effect of swelling time onto Ps. It was observed that the Ps increased with increase in swelling time upto 24 h at 25°C and thereafter, attained a constant value. This can be explained based on the fact that after 24 h the porous network of the polymer got saturated with the solvent molecules with no more accommodation for further solvent molecules.
Effect of temperature on percent swelling: It was observed that maximum Ps (6900 ± 15.27) was found at 45°C which decreased with further increase in temperature (Figure 2b). It was since beyond optimum temperature, the polymer matrix starts crumbling, thereby, leading to desorption and decreased Ps.
Sustained release of benomyl
Fungicide (Benomyl) release kinetics of Psy-cl-poly(AA)-IA: Psy-clpoly( AA)-IA exhibited 10 ± 1.15 ppm initial release of fungicide in 2 h and maximum release at the time interval of 20 h was found to be 212 ± 4.93 ppm (Figure 3). The diffusion exponent ‘n’ for Psy-cl-poly(AA)- IA has been found to be 1.16. The ‘n’ value indicated Case-II type diffusion in which the rate of diffusion is very rapid as compared with the relaxation process [32]. The Case-II type diffusion has been con irmed from the characteristic sigmoid curve obtained in the plot between Mt/M∞ versus t1/2. Gel characteristic constant ‘k’ was found to be 6.51 × 10-4 (Figure 4). Moreover, the values of initial diffusion coefficient have been found to be greater than late diffusion coefficient indicating more fungicide release in the early stages (Tables 3 and 4). Huang et al. [33] also reported Case-II diffusion at higher pH values for the release of ketoprofen using hydrogels based on cationic guar gum and acrylic acid monomer (Figures 4-6).
| Time | Sample à | Psy-cl-poly(AA)-IA |
|---|---|---|
| 2 | M | 10 |
| SE | 1.52 | |
| SD | 2.64 | |
| 4 | M | 42 |
| SE | 1.15 | |
| SD | 2 | |
| 6 | M | 95 |
| SE | 2.08 | |
| SD | 3.6 | |
| 8 | M | 115 |
| SE | 3 | |
| SD | 5.19 | |
| 10 | M | 184 |
| SE | 2.08 | |
| SD | 3.6 | |
| 12 | M | 200 |
| SE | 5.5 | |
| SD | 9.53 | |
| 14 | M | 206 |
| SE | 3.78 | |
| SD | 6.55 | |
| 16 | M | 208 |
| SE | 4.72 | |
| SD | 8.18 | |
| 18 | M | 211 |
| SE | 4.04 | |
| SD | 7 | |
| 20 | M | 212 |
| SE | 4.93 | |
| SD | 8.54 | |
| 22 | M | -- |
| SE | -- | |
| SD | -- |
Table 3: Benomyl release behaviour of Psy-cl-poly(AA)-IA. Where No. of samples used in each case=3; M=mean; SE=Standard error of mean; SD=Standard deviation; --=constant release.
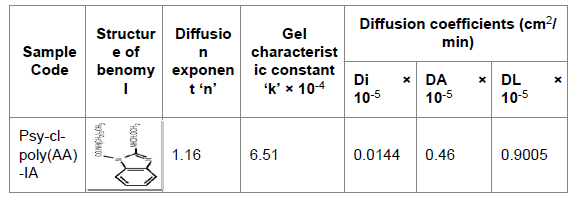
Table 4: Diffusion exponent, Gel characteristic constant and Diffusion coefficients for the release of Benomyl through Psy-cl-poly(AA)-IA at pH 6.0. Where Di=Initial diffusion coefficient, DL=late diffusion coefficient, DA=average diffusion coefficient.
The current study dealt with the synthesis of psyllium and acrylic acid-based hydrogels and their utilization in the sustained delivery of a fungicide for use in agriculture sector. It has been found that the polymer synthesized can sustain delivery of benomyl up to 20 h with release of 212 ppm. The rate of thermal decomposition of the synthesized polymer is greater as compared to that of psyllium. The diffusion exponent ‘n’ for Psy-cl-poly(AA)-IA has been found to be 1.16. The ‘n’ value indicated Case-II type diffusion. Hence the synthesized polymer can be quite important from the technological point of view.
We are thankful to Ministry of Human Resource for financing the above research and thankful to Department of Chemistry, Himachal Pradesh University, for their constructive advice during research.