
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2023)Volume 13, Issue 3
Monoclonal antibodies such as alemtuzumab are used for treatment of Multiple Sclerosis (MS) due to their high specificity and efficacy. Whilst highly effective, alemtuzumab causes autoimmune adverse events. A recent phase I clinical trial of rituximab therapy administered whenever B cell counts reached 50% of baseline levels following treatment of MS with alemtuzumab demonstrated a promising safety and efficacy profile.
The Reducing the frequency of Autoimmune adverse events in the treatment of Multiple sclerosis with alemtuzumab using B celL dEpletion (RAMBLE) trial is a phase II/III, randomised, placebo-controlled, multi-centre clinical trial conducted at five sites in Queensland, Australia. The investigational product, rituximab, is a monoclonal antibody against CD20. The study will recruit 80 people aged 18 to 55 years who have been diagnosed with MS in the last 10 years and meet outlined inclusion and exclusion criteria.
The primary objective is to demonstrate a reduction of the occurrence of autoimmune disease with administration of rituximab following treatment with alemtuzumab for MS by measuring autoimmune adverse events including thyroid disease, idiopathic thrombocytopenia and anti-GBM renal disease. The secondary objectives are to assess the safety and efficacy of this therapeutic approach as well as assess the profile of the immune repertoire of T and B cells that re-emerges.
Multiple sclerosis; Clinical trial; Alemtuzumab; Rituximab; Autoimmune disease
Alemtuzumab is a highly effective therapy for the treatment of Multiple Sclerosis (MS). In phase III clinical trials alemtuzumab reduced the risk of relapse by 51%-55% (Class I evidence) and prolonged disability by 30%-42% (Class I evidence) when compared against an active-comparator in the form of β-interferon-1a [1,2]. Alemtuzumab is administered as two courses one year apart with no further treatment being required in the majority (53%) over 8 years of follow up [3]. Despite this, alemtuzumab usage has been relatively limited due to a significant risk of autoimmune adverse events [4]. Autoimmune thyroid disease occurs in approximately one-third of people with MS and idiopathic thrombocytopenia (2%) and anti-GBM renal disease (0.5%) are seen less commonly, together with other autoimmune diseases [4]. This phenomenon is specifically seen in MS and does not occur as frequently in other indications for alemtuzumab.
Alemtuzumab is a monoclonal antibody against CD52, a lymphocyte surface marker of unknown function [5]. The antibody is lytic and causes profound, temporary lymphopenia following intravenous administration [6]. Bone marrow derived lymphocytes re-emerge rapidly and by 3 months reach 70% of pre-treatment levels [7]. One of the features of this lymphocyte repopulation is that B cells re-emerge faster than T cells [7]. In the context of a genetic melieu in people with MS that is primed for autoimmunity, it has been postulated that the extra-thymic, self-tolerisation of B cells in the absence of regulatory T cells might explain the frequent emergence of antibody-mediated autoimmune disease following treatment with alemtuzumab [8]. It has been suggested that one strategy to ameliorate the emergence of autoimmunity following alemtuzumab would be to use B cell depleting agents to repress their early re-emergence [9]. A phase I, open label, single arm clinical trial of rituximab therapy administered whenever B cell counts reached 50% of baseline levels following treatment of MS with alemtuzumab demonstrated that this approach is safe and potentially effective in mitigating the risk of autoimmunity (Class V evidence) [10].
We propose a phase II/III, placebo-controlled clinical trial with the aim of assessing the effectiveness of B cell depletion in mitigating the risk of autoimmune disease following treatment with alemtuzumab in people with MS. Given the lack of any definitive evidence for the efficacy of rituximab in this setting, a placebo was deemed to be the most appropriate comparator. The principal hypothesis to be tested is that administration of rituximab at intervals following alemtuzumab will reduce the frequency of autoimmune adverse events when compared to administration of placebo. Secondary hypotheses to be tested are that administration of rituximab after alemtuzumab will not be associated with an increased risk of adverse events and that efficacy of alemtuzumab will not be reduced. The SPIRIT reporting guidelines have been used to report the methods of this protocol [11].
Study overview
Reducing the frequency of Autoimmune adverse events in the treatment of Multiple sclerosis with alemtuzumab using B celL dEpletion (RAMBLE) trial is a phase II/III, randomised, placebo- controlled clinical trial recruiting participants diagnosed with Relapsing Remitting MS (RRMS) who are due to commence treatment with alemtuzumab [12]. This will be a multi-center study based in Queensland, Australia. The following clinical sites have been selected: Gold Coast University Hospital, Royal Brisbane and Women’s Hospital, Mater Hospital Brisbane, Sunshine Coast University Hospital and Townsville Hospital and Health Service. They will be followed for a mean of 3 years (range 2–4 years). The RAMBLE trial began enrolling participants on 3 May 2022 and is anticipated to be completed by April 2026. An outline of the study protocol is provided in Figure 1.
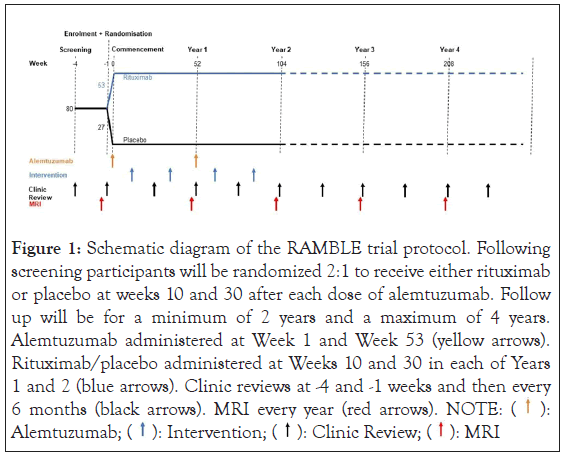
Figure 1: Schematic diagram of the RAMBLE trial protocol. Following
screening participants will be randomized 2:1 to receive either rituximab
or placebo at weeks 10 and 30 after each dose of alemtuzumab. Follow
up will be for a minimum of 2 years and a maximum of 4 years.
Alemtuzumab administered at Week 1 and Week 53 (yellow arrows).
Rituximab/placebo administered at Weeks 10 and 30 in each of Years
1 and 2 (blue arrows). Clinic reviews at -4 and -1 weeks and then every
6 months (black arrows). MRI every year (red arrows). NOTE:  Alemtuzumab;
Alemtuzumab;  MRI
MRI
Study objective
The primary objective of the RAMBLE trial is to demonstrate reduced occurrence of autoimmune adverse events following the treatment of MS with alemtuzumab through the administration of rituximab. The secondary objectives are to assess the safety (with a focus on infections) and to assess the profile of the immune repertoire of the T and B cells that re-emerge with this therapeutic approach. Safety monitoring will include measures of alemtuzumab treatment efficacy, including: time to first relapse; annualized relapse rate; Expanded Disability Status Scale (EDSS) score change [13]; change in MSIS-29 score [14]; sustained disability progression at 6 months; number of new T2 lesions MRI; and number of new Gd-enhancing lesions on MRI. Lymphocyte reconstitution will also be measured through total lymphocyte count, lymphocyte subsets and B cell subpopulation counts.
Study population
The study will aim to recruit 80 people with MS, aged 18 to 55 years who have been diagnosed with MS in the previous 10 years. Potential participants will be recruited through the MS Clinics of the five participating hospitals with a cap of 20 participants at any site. Participants will be enrolled in the study at the baseline/ screening visit provided they have provided written informed consent, meet all the inclusion criteria and none of the exclusion criteria (Table 1).
| Inclusion criteria | Exclusion criteria |
|---|---|
| Aged 18–55 years (inclusive) | Known or suspected prior autoimmune disease (other than MS) |
| Diagnosed with Relapsing Remitting MS (RRMS) by a neurologist | Any other serious co-morbidity that in the view of the investigator would preclude participation in the study |
| Diagnosis of MS meeting 2017 McDonald criteria | Pregnant (if female) |
| Diagnosed with MS within the previous 10 years | Currently lactating (if female) |
| Expanded Disability Status Scale (EDSS) score <5.0 | Unwilling or unable to use appropriate contraception for the treatment phase of the study (2 years)–male or female |
| English speaking or non-English speaking who can ensure interpreter assistance (e.g. relative or friend) to attend all visits for the duration of the clinical trial. | Recent or current history of major depression, bipolar disorder, psychosis or suicidality |
| Available to attend clinic visits | Currently or recently taking any illicit substances (including any cannabis product) |
| Willing to sign up for and comply with Bloodwatch monitoring program | Allergy to valaciclovir |
| Fully vaccinated against COVID-19 (2 standard doses plus at least one booster) | Allergy to Bactrim, Trimethoprim or Sulphur based antibiotics |
Table 1: Inclusion and exclusion criteria
Standard of care
Participants will receive alemtuzumab treatment as per standard of care. The first course will occur over days 1-5 of year one and the second course over days 1-3 of year 2. Premedication will consist of loratadine 10 mg, methylprednisolone 500 mg intravenously over 30 minutes prior to 1 L, 0.9% sodium chloride solution to run concurrently with alemtuzumab 12 mg over 4 hours. Each infusion will be monitored with hourly temperature, pulse, and blood pressure.
Treatment with alemtuzumab is associated with a 6%-8% risk of reactivation of HSV2 infection during the first month and a low risk of listeria meningitis over the same time period [15]. As part of normal care to reduce these risks, all participants will receive valaciclovir 500 mg, daily and trimethoprim/sulfamethoxazole 800 mg/160 mg three times per week (or 500 mg ampicillin in the case of allergy) for 4 weeks commencing on the first day of alemtuzumab therapy. MRI brain will be performed prior to the first course of alemtuzumab and annually thereafter in accordance with routine practice. Monthly pathology tests will include a full blood count, biochemistry, urine red cell count, urine albumin, and urinary albumin creatinine ratio, with thyroid stimulating hormone estimation every 3 months. This monitoring will be undertaken through the Bloodwatch® (RxMx®, Camperdown, Australia) monitoring scheme.
Study intervention
Investigational medicinal product: The investigational medicinal product is rituximab, a humanised, mouse, monoclonal antibody against CD20 that causes lysis of B lymphocytes [16]. Rituximab will be provided as a solution of 100 mg in 10 ml vials. Generic forms of rituximab (Ryiximols®, Roche®, France or Myorix®, Sandoz®, France) will be used. Both products have evidence of pharmacological bioequivalence to rituximab (which is no longer commercially available in Australia) and have been approved by the Therapeutic Goods Administration (TGA). Placebo will be 0.9% sodium chloride.
Dose and administration: Intravenous rituximab will be given at a dose of 100 mg/m2 of estimated Body Surface Area (BSA) based on Height (H) in cm and Weight (W) in kg according to the Du Bois formula: BSA=0.007184 x W0.425 x H0.725 [17]. Infusions will be delivered at a variable rate according to the schedules in Table 2. The investigational drug and placebo will be made up by non- blinded pharmacists according to the randomisation schedule and product information sheet for rituximab. Intervention infusions will be delivered at 10 weeks and 30 weeks after administration of alemtuzumab; provided B cell count is greater than 30% of pre- treatment levels.
| Period | 1-30 mins | 31-60 mins | 61-90 mins | 91-120 mins | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inf Rate | 50 mg/hr | 100 mg/hr | 150 mg/hr | 200 mg/hr | |||||||||||||||
| BSA (m2) | Time (min) | Dose (mg) | Rate (ml/hr) | Vol (ml) | Time (min) | Dose (mg) | Rate (ml/hr) | Vol (ml) | Time (min) | Dose (mg) | Rate (ml/hr) | Vol (ml) | Time (min) | Dose (mg) | Rate (ml/hr) | Vol (ml) | Time (min) | Dose (mg) | Vol (ml) |
| 1.5 | 30 | 25 | 33.3 | 16.7 | 30 | 50 | 66.7 | 33.3 | 30 | 75 | 100 | 50 | 0 | 0 | 0 | 0 | 90 | 150 | 100 |
| 1.6 | 30 | 25 | 31.3 | 15.6 | 30 | 50 | 62.5 | 31.3 | 30 | 75 | 93.8 | 46.9 | 3 | 10 | 125 | 6.3 | 93 | 160 | 100 |
| 1.7 | 30 | 25 | 29.4 | 14.7 | 30 | 50 | 58.8 | 29.4 | 30 | 75 | 88.2 | 44.1 | 6 | 20 | 117.6 | 11.8 | 96 | 170 | 100 |
| 1.8 | 30 | 25 | 27.8 | 13.9 | 30 | 50 | 55.6 | 27.8 | 30 | 75 | 83.3 | 41.7 | 9 | 30 | 111.1 | 16.7 | 99 | 180 | 100 |
| 1.9 | 30 | 25 | 26.3 | 13.2 | 30 | 50 | 52.6 | 26.3 | 30 | 75 | 78.9 | 39.5 | 12 | 40 | 105.3 | 21.1 | 102 | 190 | 100 |
| 2 | 30 | 25 | 25 | 12.5 | 30 | 50 | 50 | 25 | 30 | 75 | 75 | 37.5 | 15 | 50 | 100 | 25 | 105 | 200 | 100 |
| 2.1 | 30 | 25 | 23.8 | 11.9 | 30 | 50 | 47.6 | 23.8 | 30 | 75 | 71.4 | 35.7 | 18 | 60 | 95.2 | 28.6 | 108 | 210 | 100 |
| 2.2 | 30 | 25 | 22.7 | 11.4 | 30 | 50 | 45.5 | 22.7 | 30 | 75 | 68.2 | 34.1 | 21 | 70 | 90.9 | 31.8 | 111 | 220 | 100 |
| 2.3 | 30 | 25 | 21.7 | 10.9 | 30 | 50 | 43.5 | 21.7 | 30 | 75 | 65.2 | 32.6 | 24 | 80 | 87 | 34.8 | 114 | 230 | 100 |
| 2.4 | 30 | 25 | 20.8 | 10.4 | 30 | 50 | 41.7 | 20.8 | 30 | 75 | 62.5 | 31.3 | 27 | 90 | 83.3 | 37.5 | 117 | 240 | 100 |
| 2.5 | 30 | 25 | 20 | 10 | 30 | 50 | 40 | 20 | 30 | 75 | 60 | 30 | 30 | 100 | 80 | 40 | 120 | 250 | 100 |
Table 2A: Dosing regimens for first infusions of rituximab/placebo according to estimated BSA.
| Period | 1-30 mins | 31-60 mins | 61-90 mins | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inf Rate | 100 mg/hr | 200 mg/hr | 300 mg/hr | ||||||||||||
| BSA (m2) | Time (min) | Dose (mg) | Rate (ml/hr) | Vol (ml) | Time (min) | Dose (mg) | Rate (ml/hr) | Vol (ml) | Time (min) | Dose (mg) | Rate (ml/hr) | Vol (ml) | Time (min) | Dose (mg) | Vol (ml) |
| 1.5 | 30 | 50 | 66.7 | 33.3 | 30 | 100 | 133.3 | 66.7 | 0 | 0 | 0 | 0 | 60 | 150 | 100 |
| 1.6 | 30 | 50 | 62.5 | 31.3 | 30 | 100 | 125 | 62.5 | 2 | 10 | 187.5 | 6.3 | 62 | 160 | 100 |
| 1.7 | 30 | 50 | 58.8 | 29.4 | 30 | 100 | 117.6 | 58.8 | 4 | 20 | 176.5 | 11.8 | 64 | 170 | 100 |
| 1.8 | 30 | 50 | 55.6 | 27.8 | 30 | 100 | 111.1 | 55.6 | 6 | 30 | 166.7 | 16.7 | 66 | 180 | 100 |
| 1.9 | 30 | 50 | 52.6 | 26.3 | 30 | 100 | 105.3 | 52.6 | 8 | 40 | 157.9 | 21.1 | 68 | 190 | 100 |
| 2 | 30 | 50 | 50 | 25 | 30 | 100 | 100 | 50 | 10 | 50 | 150 | 25 | 70 | 200 | 100 |
| 2.1 | 30 | 50 | 47.6 | 23.8 | 30 | 100 | 95.2 | 47.6 | 12 | 60 | 142.9 | 28.6 | 72 | 210 | 100 |
| 2.2 | 30 | 50 | 45.5 | 22.7 | 30 | 100 | 90.9 | 45.5 | 14 | 70 | 136.4 | 31.8 | 74 | 220 | 100 |
| 2.3 | 30 | 50 | 43.5 | 21.7 | 30 | 100 | 87 | 43.5 | 16 | 80 | 130.4 | 34.8 | 76 | 230 | 100 |
| 2.4 | 30 | 50 | 41.7 | 20.8 | 30 | 100 | 83.3 | 41.7 | 18 | 90 | 125 | 37.5 | 78 | 240 | 100 |
| 2.5 | 30 | 50 | 40 | 20 | 30 | 100 | 80 | 40 | 20 | 100 | 120 | 40 | 80 | 250 | 100 |
Table 2B: Dosing regimens for subsequent infusions of rituximab/placebo according to estimated BSA.
Study related procedures
Screening and consenting process: Potential participants will be screened 4 weeks prior to the planned date of the first course of alemtuzumab. All participants will be required to provide written, informed consent. Participants will be required to demonstrate a comprehensive understanding the trial and be provided an opportunity to have questions answered. Principal investigators will be responsible for the consenting process. The patient information and consent form includes opt-in consent to the use of participant samples for ancillary laboratory studies. The following will be undertaken at the screening visit: comprehensive review of medical history; including relapse history; past medical and surgical history; medications and allergies; general and neurological examination; EDSS; and Hospital Anxiety and Depression Score [18]. MSIS-29 will be performed at the enrolment visit.
Randomisation and blinding: Participants and treating clinicians will be blinded as to whether the participant is receiving rituximab or placebo. At the screening visit, participants will be assigned a unique Participant Identification Number (PIDN). At the enrolment visit, after eligibility is confirmed, participants will be randomly assigned to intervention or placebo groups using the randomisation feature of REDCap® (Vanderbilt University, Nashville, TN, USA). Participants will be randomised to treatment with rituximab or placebo at a 2:1 ratio. Randomisation will be stratified in blocks of 6 based on age (18-30, 31-43 and 44-55) and sex (male or female), using a computer-generated randomisation table by our statistician (Professor Jing Sun). Randomisation outcomes will only be visible through REDCap® to site pharmacists and two central coordinators who will undertake the randomisation procedure and approve dosing based on B cell counts that only they will have access to. Both rituximab solution and 0.9% sodium chloride (placebo) are colourless fluids and will be indistinguishable. The randomisation code for an individual participant may only be unblinded in emergency situations, where the site principal investigator decides a participant cannot be adequately treated without knowing their treatment allocation and following discussion with the Chief Investigator.
Monitoring visits: Monitoring visits will occur every 6 months where there will be a review of relapse history, intercurrent illness, medications, EDSS and adverse events. All principal investigators will have current certification for EDSS scoring with Neurostatus® (University Hospital Basel, Switzerland). Monitoring for the primary outcome measure will occur clinically and through the Bloodwatch® monitoring platform. Adverse events can be reported through the RAMBLE REDCap® database by any investigator involved in the study at any timepoint.
Data collection and integrity: Data will be collected in an electronic case report form created using a specifically designed REDCap® database. Participant confidentiality will be maintained using a unique PIDN with no identifiable information used in the database. Source documents will be paper based or electronic medical records at each study site subject to local security and access arrangements (e.g. password protected access to Queensland Health electronic medical records). The REDCap® database has user level password protection and access will be restricted to principal investigators and their delegates at each site. Access to record will be site specific. Only de-identified data will be made publicly available. Investigators will have full access to the data collected.
Stopping rules
A safety review board will monitor unblinded summary data for adverse events every 3 months. The study will be stopped if there is a statistically significant excess of adverse events associated with rituximab administration. The study will also be suspended immediately in the event of a serious adverse event occurring that is thought to be associated with the administration of rituximab. In the event of a significant, known infusion related reaction to rituximab (e.g. anaphylaxis) administration of rituximab/placebo for that individual participant will be suspended.
Loss to follow up
Participants will be actively encouraged to remain in the trial. Participants will not be considered lost to follow until all routes of contact have been exhausted. If a participant wishes to drop out for any reason, site staff will negotiate with the participant to reduce trial duties with a focus on continued collection of data relating to the primary outcome.
Statistical analyses
The primary outcome measure for this study will be time to onset of autoimmune adverse event modelled using a Cox Proportional Hazards time to event analysis [19,20]. Analysis will be performed on an intention-to-treat basis and will include potential baseline confounders (e.g. age, sex, clinical site). The primary analysis will be based on observed data only, but a sensitivity analysis where missing data is imputed using maximum likelihood imputation methods will be undertaken. The primary outcome of this trial will be met if the hazard ratio of autoimmune adverse events in the treatment arm is significantly lower than placebo.
Other secondary outcomes (e.g. change in EDSS, new T2 and Gd- enhancing MR imaging lesions) at 1 and 2 years will be assessed using linear regression analysis provided assumptions are met, or if not, non-parametric methods will be used. Other adverse events will be compared using frequencies and chi-squared statistics. Efficacy in terms of MS outcomes will be analysed using time to event analysis (Cox Proportional Hazards method) for time to first relapse and 6 month sustained disability progression, including the factors listed above for the primary outcome analysis in the model. Any effect of laboratory measures (lymphocyte subsets) and baseline characteristics will be explored using regression analysis. All statistical analyses will be conducted using STATA® v16 software (StataCorp®, College Station, TX, USA).
Sample size calculation
A previously published, sample size calculation based on the phase I study suggested that a trial of 80 participants (40 in each arm) would have 80% power with p<0.05 to detect a reduction in autoimmune adverse events from 40% down to 10% [10].
The study is in the preliminary stages of collecting data and results have not yet fully been collected. The submission was for the trail protocols papers.
The RAMBLE trial has been approved by the human research ethics committees (HRECs) of Gold Coast Hospital and Health Service (HREC/2021/QGC/75077) and Griffith University (2021/807). Any amendments to the study protocol will require the approval by these HRECs. The study is currently on protocol v3.0 dated 10 June 2022 following the approval of Amendment 1 on 16 August 2022. The RAMBLE trial unique trial number (UTN) is U1111- 1284-6567. The trial was registered with the Australian and New Zealand Clinical Trials Registry (ACTN12621001502820p) on 4 November 2021 and a Clinical Trial Notification for rituximab, methylprednisolone and loratadine has been processed by the TGA (CT-2021-CTN-04368-1 v2). In accordance with international publication and regulatory requirements fully de-identified aggregate data and where necessary raw data will be made available for independent verification of results and regulatory processes. The results of this trial will be in a leading clinical journal. Participants who have elected to provide contact details on their consent form will be provided with a lay summary of the results on completion of the trial.
The RAMBLE trial is being sponsored by Griffith University, Queensland, Australia and has been funded by a donation from the Brazil Family Foundation and a research grant from MS Australia (Project Grant 21-2-060). There has been no commercial sponsorship for this trial. The project is managed through a steering committee consisting of the Chief Investigator (Professor Simon Broadley) and two advisors (Professors Michael Levy and Helmut Butzkueven). Day-to-day operations are managed through quarterly investigator meetings consisting of the principal investigators and delegates for each site. Compliance with the protocol will be monitored through a monthly audit of the RAMBLE REDCap® database on site visits to each site annually. An independent Safety Review Board, consisting of two neurologists and one immunologist, who are not connected with the project, has been formed. Costs of care for adverse events will be covered through the Australian state-backed health care system in the usual way. In the event of compensation being required for those that suffer harm as a result of the trial, Griffith University holds a comprehensive medical insurance policy with Uni-mutual®, Sydney, Australia.
The study design was developed by SAB, PAMcC, MB, SBl, ZL, LC, JB, SA, KW, SHC, RW, MP, VC, FM-C, SJS, CO, CC and MN. The first draft of the manuscript was prepared by MN and CC. All authors have reviewed and approved the final version of the manuscript. All authors meet the National Health and Medical Research Council (Australian Research Council) criteria for authorship.
This trial is supported by grants from the Brazil Family Foundation (Tel: +61 7 3217 8400) and MS Research Australia (Tel: +61 1300 010 158).
None declared
This study has been approved by the Gold Coast Hospital and Health Service HREC in January 2022 as the lead site. HREC Approval Number HREC/2021/QRC/75077. The study has also been reviewed and approved by the HREC of Griffith University (2021/807) and the Governance Review Boards of the participating sites.
This trial has been registered with the Australian New Zealand Clinical Trials Registry (ACTRN1262100150282p). On completion of the study access to complete de-identified patient level data will be provided through a suitable sharing platform such as Vivli (Multi-regional Clinical Trials, Harvard, MA, US).
Chief Investigator:-Simon Broadley (Gold Coast University Hospital)–PI
School of Medicine and Dentistry, Gold Coast Campus, Griffith University QLD 4222, Australia. Email: simon.broadley@griffith. edu.au
Simon Arnett (Gold Coast University Hospital)-AI
Pamela McCombe (Royal Brisbane and Women’s Hospital)–PI
Zara Ioannides (Royal Brisbane and Women’s Hospital)–AI
Stefan Blum (Mater Hospital Brisbane)–PI
Andrew Swayne (Mater Hospital Brisbane)–AI
Mike Boggild (Townsville University Hospital)–PI
Joshua Barton (Sunshine Coast University Hospital–PI
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ntiamoah M, Camarda C, Osborne C, Sanchez SJ, Marple-Clark F, Cottam V, et al. (2023) Study Protocol for a Phase II/III, Randomised,Placebo-Controlled Clinical Trial of Reducing the Frequency of Autoimmune Adverse Events in the Treatment of Multiple Sclerosis with Alemtuzumab using B Cell Depletion: The RAMBLE Trial. J Clin Trials. 13:528.
Received: 19-Mar-2023, Manuscript No. JCTR-23-22277; Editor assigned: 21-Mar-2023, Pre QC No. JCTR-23-22277 (PQ); Reviewed: 04-Apr-2023, QC No. JCTR-23-22277; Revised: 11-Apr-2023, Manuscript No. JCTR-23-22277 (R); Published: 18-Apr-2023 , DOI: 10.35248/2167-0870.23.13.528
Copyright: © 2023 Ntiamoah M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.