Virology & Mycology
Open Access
ISSN: 2161-0517
ISSN: 2161-0517
Short Communication - (2022)Volume 11, Issue 3
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China, mid-December 2019. In March 2020, World Health Organization declared the coronavirus disease 2019 (COVID-19) a pandemic [1,2]. Healthcare Workers (HCW), in the frontline, play a significant role in accompanying patients infected with coronavirus and are also at a higher risk of infection [3,4]. After SARS-CoV-2 contact, antibody titers are vital biomarkers not only for their defensive effectiveness, likewise for the assessment of humoral response. This is specifically pertinent due to the paucity of information concerning post-infection immunity [5,6].
More recently, there has been an astonishing determination to identify COVID-19 new treatments, diagnostic tests, and vaccines against the virus [7,8]. Antibodies detailed to COVID-19 are significant for removal and clearance of the virus and are measured by in vitro neutralization proves, after vaccination [9,10].
After HCWs were knowledgeable about the COVID-19 antibody serological study and support was obtained from the hospital's ethics committee (IRB approval number: E.21078233) to examine the incidence of SARS-CoV-2 antibodies among HCWs of the ICU after Pfizer-BioNTech vaccine administration. The HCWs were doctors (n=15), nursing staff (n=43), operational assistants (n=5), and administrative staff (n=2).
The study population was divided into two groups: IgG antibodies greater than 15.000 (group one) and less than 15.000 (group two). It was evaluated sex, age and symptoms as clinical and demographic information after the third administration of the vaccine.
For the quantitative and qualitative purpose to determine IgG antibodies to the receptor binding domain of the spike protein of SARS-CoV-2 it is used the SARS-CoV-2 IgG II Quant assay of human serum and plasma.
Statistics investigation was accomplished using the chi-square test for categorical variables and the pearson's correlation for continuous variables. For the graphical analysis, Boxplot plots and scatter plots were executed. Statistics evaluation was implemented using SPSS for Windows version 25.0.
A total of 65 HCWs were included in this study, 36 males and 29 females. Group 1 is consisted by 51 individuals and had IgG antibodies greater than 15.000. Group 2 has 14 individuals and IgG antibodies less than 15.000.
There was a seroprevalence of 78.5% in group 1 and 21.5% in group 2. The seroprevalence of COVID-19 immunoglobulin G (IgG) antibodies after the third dose of vaccine administration is represented in Figure 1.
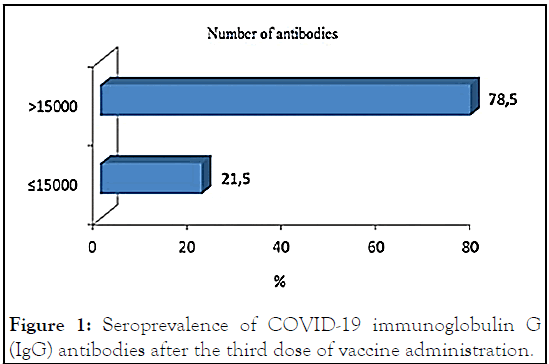
Figure 1: Seroprevalence of COVID-19 immunoglobulin G (IgG) antibodies after the third dose of vaccine administration.
Group 1 consisted mainly of male individuals (68.6%), unlike group 2 which consisted mainly of female individuals (92.9%), as shown in Figure 2. This was statistically significant (p<0.001).
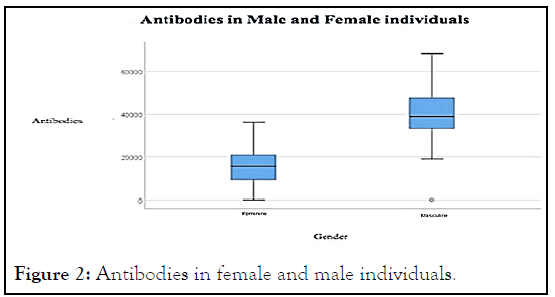
Figure 2: Antibodies in female and male individuals.
In Group one the average age was 40 years and was mostly male. In group two, the standard age was 33 years and was mainly female. In group one, furthermost HCWs did not have other diseases (55%); however, in group two, most had associated pathologies (60%). The most recurrent were asthma, hypertension and hypothyroidism.
We also concluded that most of group 1 had more symptoms (94.1%) and a minority of group 2 had less symptoms (14.3%), with statistical significance (p<0.001). After the administration of the vaccine, group 1 had more symptoms (p<0.001).
These symptoms were myalgias (50.8%), adenopathies (35.4%), fever (27.7%), asthenia (24.6%), vomiting (12.3%), diarrhea (10.8%), chills (6.2%), epigastric pain and arthralgia (1.5%). Figure 3 shows the symptoms after the administration of the third dose of vaccine.
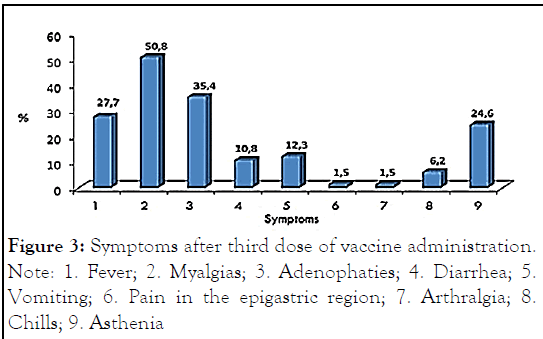
Figure 3: Symptoms after third dose of vaccine administration.
Note: 1. Fever; 2. Myalgias; 3. Adenophaties; 4. Diarrhea; 5.Vomiting; 6. Pain in the epigastric region; 7. Arthralgia; 8. Chills; 9. Asthenia
One of the main aspects for considerate viral clearance and vaccination efficacy is humoral and cell-mediated reaction against SARS-CoV-2. Learning the kinetics of SARS-CoV-2 antibodies is useful and advantageous not only to analysis the effect of the COVID-19 infection in the community but also to screen trends in the virus spread. Casado et al. confirmed that HCWs gotten satisfactory cellular and humoral reply after a unique dose of an mRNA vaccine [11]. Regarding the present study, it was concluded that more participants showed antibody titers greater than 15.000, and this group consisted mainly of male individuals (68.6%) that had more symptoms after vaccine administration (p<0.001). As for the second group (IgG antibodies less than 15.000) it consisted mainly of female individuals (92.9%) with a small minority having less symptoms (p<0.001).
In an Israeli study, concerning dialysis patients, antispike (anti-S) IgG antibody titers were assessed before and after a third BNT162b2 dose in individuals aged 60 years and older. They concluded that there was a significant increase in IgG titers, as can also be seen in our study. Though collecting evidence suggests that IgG response is a correlate of disease protection, cellular immunity has also been suggested to play an important role in protecting against SARS-CoV-2 [12]. In another study, also regarding dialysis patients, who included 69 individuals, they also established that a third dose increased antibody levels with acceptable reactions to the vaccine [13]. In a 2022 study it was determined that most common systemic side effects following vaccine administration were fatigue (30.18%), headache (28.89%), muscle pain (25%), chills (19.67%), fever (16.57%), and joint pain (9.20%) [14]. As for the symptoms reported by the participants in our study, they were myalgias (50.8%), fever (27.7%), adenopathies (35.4%), asthenia (24.6%), vomiting (12.3%), diarrhea (10.8%), chills (6.2%), epigastric pain and arthralgia (1.5%), with most of these overlapping with reported symptoms in other studies. Another recent study established that the cornerstones of guaranteeing the best protection are current vaccination strategies, alongside with preventive measures, for all individuals. The most efficient strategy to combat the current COVID-19 pandemic is represented by active immunization [15].
Given the small size of our population, we consider this to be a limitation of our clinical study. The study determined that male professionals with a superior number of IgG antibodies developed the most symptoms. The most common symptoms were myalgias, adenopathies, and asthenia. It is essential to note that additional analyses are required on this question and that this is a part of research where data is still quite limited and inadequate, particularly concerning the fourth dose of the vaccine.
Consent was obtained by all participants in this study. Ethics Committee for Health and Scientific and Research Committee of the Health Service of the Autonomous Region of Madeira, EPERAM issued approval E.21078233. Medical Ethical Committee approved the study on June 14, 2021.
Citation: Lemos CIG, Graça C, Gouveia C, Real FC, Ferreira S, Placido C, et al. (2022) Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies among Healthcare Workers after a Third Dose of Vaccine in an Intensive Care Unit: A Short Communication. 10:230.
Received: 13-May-2022, Manuscript No. VMID-22-17496; Editor assigned: 18-May-2022, Pre QC No. VMID-22-17496 (PQ); Reviewed: 01-Jun-2022, QC No. VMID-22-17496; Revised: 06-Jun-2022, Manuscript No. VMID-22-17496 (R); Published: 14-Jun-2022 , DOI: 10.35248/2161-0517.22.11.230
Copyright: © 2022 Lemos CIG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.