Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2025)Volume 15, Issue 1
The associations among sunshine exposure, D supplementation and serum vitamin D concentrations are well known. Guidelines for dietary intake and serum concentrations are in- tended to limit the population risk of vitamin D deficiency. Present recommendations have been widely unsuccessful in achieving vitamin D sufficiency in childhood, adolescence and adulthood. Specific age-dependent seasonal D distribution curves do not exist, but they would allow an evidence-based estimate of vitamin D requirements for boys and girls in different stages of development. In a secondary analysis of the first wave (May 2003-May 2006) of the nationwide representative German KiGGS Study (Study on the health of children and adolescents in Germany), we reanalysed data from 10,015 participants to investigate the seasonal variations in serum vitamin D concentrations for children and adolescents within two-year age intervals to increase our understanding of the relationship between serum vitamin D concentrations and child development in both sexes. Except for the first year of life, when infants are fully supplemented with 400 IU vitamin D, we found seasonal and age-dependent vitamin D distribution patterns for all age groups. The age groups between 2 and 9 years (grouped in 2-3, 4-5, 6-7 and 8-9 years) showed a positive deviation and the age groups between 10-15 years showed a negative deviation from the mean curve over all age groups. The time frame between 10-15 years is associated with puberty and the lower serum concentrations may indicate a higher conversion rate of 25(OH) vitamin D into 1,25(OH)2 vitamin D. The pubertal decrease in serum vitamin D concentrations between boys and girls differs significantly in time, length and magnitude. As puberty starts and ends earlier in girls than in boys, this difference may suggest that puberty is possibly among others a causal factor for the observed drop in serum vitamin D concentrations. Vitamin D deficiency especially in critical growth periods may hamper bone and immune health which has so far not been sufficiently considered. The dilemma of multiple factors and their influence on distribution curves can probably only be solved by using machine learning programs, which may be better suited to take multiple determinants into account to provide reliable data to achieve adequate vitamin D supplementation
Vitamin D; Deficiency; Age; Childhood; Adolescence; Puberty
The answer to the question of an age-appropriate seasonal vitamin D requirement, as well as the question of optimal serum vitamin D concentrations under varying conditions of solar exposure, genetics, age, sex and environment, can largely not be answered by available evidence [1]. Guidelines and recommendations by governments and nongovernmental institutions differ in their conclusions and their updates seem to rest in a stalemate [2]. National representative surveys monitoring vitamin D status and a closer look at factors influencing that status seem to be necessary for updating current guidelines.
In a secondary analysis using data from the representative German KiGGS Study (Study on the health of children and adolescents in Germany), we investigated the seasonal variations in serum vitamin D concentrations of children and adolescents at two-year-age intervals to better understand the time frames and determinants of the interactions between serum vitamin D concentrations and child development.
Seasonal data from 10,015 study participants aged between 0 and 17 years were taken from the nationwide first wave of the KiGGS study. The data were collected between May 2003 and May 2006. Methods and initial results have been reported elsewhere [3]. For the secondary analysis, we used the RKI data divided into smaller 2-year age groups (0-1, 2-3, 4-5, 6-7, 8-9, 10-11, 12-13, 14-15 and 16-17 years) to obtain a closer view of the age-dependent vitamin D distribution curves. The age group of 0-1 years is underrepresented compared to the other age groups, which was considered in the analysis. Serum vitamin D status was first averaged as a function of calendar months (January to December) and plotted over 0-17 years (Figure 1). The characteristics of sex and age groups were examined for differences in their distributions over age and months. The two sample t test was used to assess statistically significant differences.
Age composition and age-specific participant numbers in the KiGGS Study (May 2003-May 2006) are presented in Table 1.
| Age groups (Years) | Number of KiGGS study participants |
|---|---|
| 0-1 | 337 |
| 2-3 | 914 |
| 4-5 | 1063 |
| 6-7 | 1228 |
| 8-9 | 1338 |
| 10-11 | 1338 |
| 12-13 | 1337 |
| 14-15 | 1314 |
| 16-17 | 1145 |
| Total number of participants | 10015 |
Table 1: Age groups and participant numbers in the first wave of the KiGGS study (May 2003 - May 2006).
Serum vitamin D concentrations largely depend on seasonal changes in UV-B exposure for endocrine synthesis [4]. Other factors, such as ethnicity, genetics, skin type, diet, weight (overweight, obesity), lifestyle (indoor versus outdoor) and the developmental age of children and adolescents, possess modulating effects [5]. In temperate zones, seasonally collected serum vitamin D (25(OH)D) concentrations present like a sinus curve under cyclic day/night variations of solar exposure. In Germany, the lowest serum vitamin D concentrations occur in January and February and the highest concentrations occur between August and October. Mean vitamin D sufficiency (generally defined as values of ≥ 50 nmol/l or ≥ 20 ng/ml) cannot be achieved by all children and adolescents in the summer months without supplementation. Winter lows of approximately 20 nmol/l (8 ng/ml) in January and February almost preclude the achievement of naturally acquired vitamin D sufficiency in the summer. The highest mean serum vitamin D values (Figure 1), shown by age groups, reached a plateau-like peak at different heights mainly between July and September. Only infants who received vitamin D supplementation had serum vitamin D concentrations in the sufficiency range of between 70 nmol/l-80 nmol/l and those concentrations are largely independent of seasonal changes [6]. The distribution curves for the 2-9-year-olds (green and blue) and the 16-17-year olds (red) slightly exceed the curve for the overall means (black curve). The yellow curves show vitamin D distribution changes for the age groups of 10-15 years, categorised into 10-11, 12-13 and 14-15 years. All values in these three age groups undercut the mean.
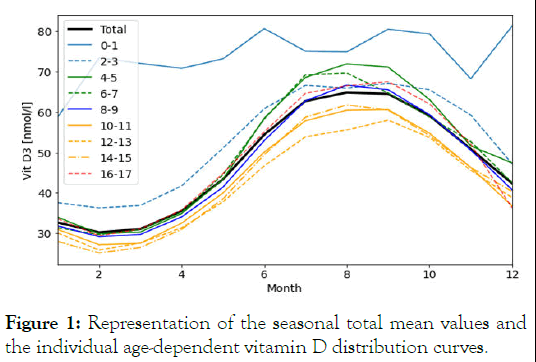
Figure 1: Representation of the seasonal total mean values and the individual age-dependent vitamin D distribution curves.
The flattening of the vitamin D distribution curves of 10 to 15 year-old girls and boys falls into the time frame of puberty with its pubertal growth spurt and its associated increased bone mineralisation [7]. Biomarkers of bone metabolism activity studied by Rand et al. show the highest activity between 11-12 years in girls and 13-14 years in boys [8]. Aksnes and Aarskog demonstrated that the two quotients of 25(OH)D and 24,25(OH)2D are inversely associated with 1,25(OH)2D status during puberty pointing to a higher vitamin D requirement [9]. In particular, the curve between 12 and 13 years, which is associated with the most intense period of puberty for both sexes, shows the highest flattening. We postulated that the earlier onset of puberty in girls and the later onset and longer duration of puberty in boys might allow us to infer causality for the drop in vitamin D values in both sexes. Looking for puberty dependent significant differences, we categorised the serum vitamin D distribution curves by sex across all age groups (Table 2).
| Age groups by years | t value | P value |
|---|---|---|
| 0-1 | 0.46 | 0.65 |
| 2-3 | 1.19 | 0.23 |
| 4-5 | -0.23 | 0.82 |
| 6-7 | 0.35 | 0.73 |
| 8-9 | 0.52 | 0.6 |
| 10-11 | 5.01 | 0 |
| 12-13 | 4.87 | 0 |
| 14-15 | -1.02 | 0.31 |
| 16-17 | -5.76 | 0 |
| All age groups | 1.63 | 0.1 |
Table 2: Results of the two-sample t test rounded to two decimal places. The distributions per age group for boys and girls were tested.
The separation of all study participants by sex only showed a significant difference between boys and girls between 10-11, 12-13 and 16-17 years (Figure 2).
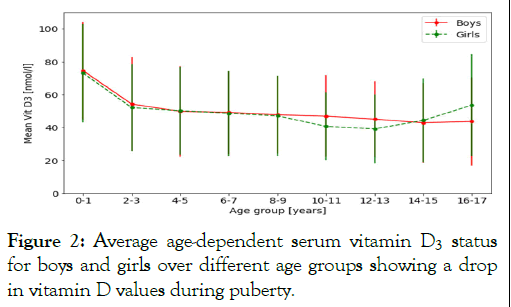
Figure 2: Average age-dependent serum vitamin D3 status for boys and girls over different age groups showing a drop in vitamin D values during puberty.
Figure 2 shows the sex differences in the pubertal time frame from 9 to 17 years. Serum vitamin D concentrations decrease in both sexes during puberty, with girls showing a more pronounced course in comparison to boys. Puberty in girls is associated with a shorter but higher impact on serum vitamin D values and recovery after the age of 15 years.
The flattened yellow serum vitamin D distribution curves (Figure 1) lie within the time frame of puberty with increased vitamin D requirements and consumption associated with the pubertal growth spurt [9]. During puberty, more inactive vitamin D (25(OH)D) is converted into active vitamin D (1,25(OH)2D) [7]. In comparison to girls, puberty in boys shows a smaller but longer lasting impact on serum vitamin D values.
Seasonal variations in latitude-dependent solar exposure and differences in dietary intake, metabolism, age and lifestyle factors limit the effectiveness of existing guidelines for vitamin D intake [2,10]. The highest serum vitamin D concentrations in temperate climate zones are reached in summer and the lowest in winter. Serum vitamin D concentrations are measured as 25 hydroxyvitamin D (25(OH)D), which serves as a biomarker for serum vitamin D status and is the inactive precursor of the biologically active 1,25(OH)2D. In most guidelines, vitamin D deficiency begins at serum concentrations of <50 nmol/l (<20 ng/ml) [11]. Vitamin D deficiency is relevant to health outcomes. At these and lower concentrations, impairment of bone mineralisation and immune functions may occur [12,13]. Vitamin D also has a modulatory effect on a variety of immune cells, including activated CD4 and CD8 T lymphocytes, B lymphocytes, macrophages and dendritic cells [14,15]. Serum 25(OH)D concentrations are inversely related to the levels of C Reactive Protein (CRP), Interleukin-6 (IL-6) and Tumour Necrosis Factor-α (TNF-α) [16]. Vitamin D supplementation has been shown to decrease Nuclear Factor kappa B (NF-kB) activity, interferon-γ, TNF-α and pro-inflammatory cytokines IL-6 and IL-12 and increase anti-inflammatory IL-10 levels [17,18]. By using leukocytes from normal-weight African Americans aged 14-19 years, Zhu et al. demonstrated with a DNA methylation study that vitamin D deficiency with serum 25(OH)D concentrations of ≤ 25 nmol/l resulted in statistically significant genomic and epigenomic changes in leukocyte DNA compared to controls with serum concentrations of >75 nmol/l [19]. At serum concentrations of >75 nmol/l, counter regulation of the parathyroid gland ceases. Some experts therefore recommend serum concentrations of >75 nmol/l (>30 ng/ml) as optimal for the general population to prevent bone mineralisation and immunological defects [20]. The actual vitamin D requirement in children and adolescents to achieve vitamin D sufficiency in the absence of endogenous synthesis via solar exposure is estimated to be 400 IU (10 µg/day) for infants and 800 IU/day (20 µg/day) for all other age groups (DGE-German Society for Nutrition). With estimated intakes of approximately 1 µg/day-2 µg/day (40-80 IU) in children and approximately 2 µg/day-4 µg/day (80-160 IU/day) in adolescents, vitamin D sufficiency cannot be achieved without solar exposure. The question of how much vitamin D children and adolescents need to achieve sufficient serum vitamin D concentrations across all seasons and developmental stages is complex and has not been adequately addressed. Different intake sources of vitamin D, cyclic seasonal variations of solar radiation, different genetic factors, differences in socioeconomic status and varying lifestyle factors limit the usefulness of present guidelines on vitamin D intakes and requirements. It may therefore not be surprising that the guidelines of professional societies show large variations in their recommendations and have not been useful in eradicating vitamin D insufficiency. Our study shows that increased vitamin D requirements have to be met in puberty, which has so far not been sufficiently taken into consideration. Saneifard et al., recently reported that puberty poses an additional risk factor for vitamin D deficiency, especially in girls and obese children. According to a study of pubertal growth velocity during 1965-1970 conducted by Aksglaede et al., the pubertal growth spurt begins at 10.2 years for girls and at 11.8 years for boys. The highest growth rate is reached at age 12 and 14.2 years of age in girls and boys, respectively. The highest calcium intake is reached 12.5 and 14 years in girls and boys, respectively. These changes are reflected in the age dependent vitamin D distribution curves. In this context, we must keep in mind that vitamin D also increases calcium absorption in the gut for optimalization of bone mineral density. With an analysis of 17 case control and 6 cross-sectional studies compared to 5000 controls, Yang et al., showed that serum 25(OH)Vit-D concentrations were lower (25(OH)Vitamin D ≤ 50 nmol/l) in paediatric patients with fractures. Moore et al. reported similar results in an Irish prospective case control study with a 5-year follow-up. The increased vitamin D deficiency risk, together with the fact that puberty is the most important time for building a healthy skeleton, supports vitamin D monitoring, especially in active developmental phases. Even intermittent seasonal vitamin D deficiency seems to influence bone development, later osteoporosis risk, immunological functions, adiposity and atherosclerosis risks. The importance of seasonal differences in bone mineralisation was demonstrated in adults by iliac crest bone biopsies, calcium absorption data and serum vitamin D metabolites in 121 patients attending an osteoporosis clinic in Adelaide, Australia. Osteoid thickness was higher in winter months when 25(OH)D concentrations were low and lower in summer months when 25(OH)D concentrations were high. Studies by Priemel et al. performing iliac crest biopsies across all age groups confirm these findings by showing that no osteoid in bone could be found above a minimum serum 25(OH)D value of ≥ 75 nmol/l. Vitamin D deficiency inhibits osteoblast activity and calcium uptake via the intestine.
1,25(OH)2D (calcitriol) activated via 1-hydroxylation of 25(OH)D (calcidiol) is correlated with the accumulation of bone mass during the pubertal growth spurt [7]. Non-white and obese children and adolescents carry a higher risk of vitamin D deficiency. They are more likely to develop bowlegs and Blount disease than toddlers. Rosengren et al. pointed to a 40-year decline in bone density and bone quantity in children and adolescents aged 7 to 15 years. The cause cannot be understood.
If this may be due to increasing adiposity, less time spent outdoors and changes in dietary habits with reduced consumption of dairy products or other factors needs to be clarified in the interest of preventing vitamin D deficiency in children and adolescents.
Vitamin D deficiency is ubiquitous in all age groups, posing a threat to bone and immune health. Present vitamin D guidelines have not led to an improvement in vitamin D deficiency in German children and adolescents. Furthermore, some guidelines do not even recommend supplementation for “healthy” children older than 2 years who do not reach vitamin D sufficiency. Guidelines seem to rest in a stalemate, as the dimensionality of influencing factors and their different statistical weights cannot be sufficiently caught and defined in guidelines for practical use. General population testing is not recommended due to the associated high costs. A way out of this dilemma could be the bloodless assessment of vitamin D deficiency risks by using machine learning programs with different embedded risk profiles (e.g., age groups, obesity) based on existing datasets. If sufficiently validated, machine learning programs can be expected to reduce the need for blood testing, providing individual and general health and economic advantages. Some investigators have taken the first steps by using vitamin D Machine Learning Programs (MLPs) in conjunction with vitamin D concentrations in defined diseases.
The original data were transferred from the RKI to the HRW for secondary analysis. Both authors declare that no funds, grants or other support were received during the preparation of the manuscript.
JH is patent holder of a vitamin D formulation, co-founder of nutrelligence and has received speaker and research honoraria from ALIUD and HiPP. ASN declares no competing interests.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Anne Stockem Novo and Jurgen Hower. The first draft of the manuscript was written by Jurgen Hower. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Votes of approval for the KiGGS Study of the German Ministry of Heals (RKI) have been obtained from the Ethics Commission of Charite University Hospital Berlin and Germany’s Federal Commissioner for Data Protection.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hower J, Novo AS (2025) Seasonal, Age and Sex-dependent Variations in Serum Vitamin D Concentrations in Children and Adolescents and their Relevance for Future Health. Trans Med. 15:340.
Received: 21-Jan-2024, Manuscript No. TMCR-24-29265; Editor assigned: 23-Jan-2024, Pre QC No. TMCR-24-29265 (PQ); Reviewed: 06-Feb-2024, QC No. TMCR-24-29265; Revised: 05-Feb-2025, Manuscript No. TMCR-24-29265 (R); Published: 12-Feb-2025 , DOI: 10.35248/2161-1025.25.15.340
Copyright: © 2025 Hower J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.