
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Short Communication - (2020)Volume 3, Issue 2
In late 2019, a cluster of cases of viral pneumonia of unknown etiology was reported in Wuhan, Hubei Province, China [1]. This new viral pneumonia, COVID-19 (Coronavirus Disease 2019), caused by the novel SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2), spread rapidly and developed into a global pandemic [2].
Currently in Italy more than 200,000 cases have been recorded with a high prevalence in some northern regions and a substantial low incidence in the southern regions [3].
COVID-19 is currently diagnosed through detection of SARSCoV- 2 in upper and lower respiratory specimens by molecular tests for viral RNA, such as RT-PCR [4].
However, these methods are dependent on the time-window of viral replication, low viral titer, and subject to incorrect sample collection. These factors can potentially cause low positivity rates, especially>2 weeks after onset, thereby limiting their usefulness [4].
Anti-SARS-CoV-2 antibodies may represent a tool that can help close the RT-PCR negative gap and significantly increase diagnostic sensitivity for COVID-19 [5].
Moreover, it becomes more and more evident that, notwithstanding the importance of the diagnostic role of SARS-CoV- 2 antibodies testing, its epidemiologic potential to evaluate a population's immunization state is increasingly important.
Fully automated analyzers characterized by high-throughput and low complexity have helped us to use serological testing more accurately during the different phases of the disease.
In this paper we report the experience of Varelli Diagnostic Institute on 9,480 serum samples from different Italian private laboratories collected from April 29th to May 18th, 2020.
All samples were processed for the presence of Anti-SARS-CoV-2 IgG antibodies with an ARCHITECTi2000 (Abbott) CLIA automatic analyzer. Results are expressed as arbitrary unit (index) [6] with a cutoff value of 1.40. Microsoft Excel and R were used for data analysis and illustration.
Sex information was available for 9,363 patients (98.8% of total) showing equal distribution for males and females (50.1% and 49.9% respectively). No sexual imbalance was found in disagreement with what reported elsewhere [7].
Age information was available only for 7,237 samples (76.3%), therefore it was not possible for us evaluate the age distributions for a significant fraction of our population, particularly for patients from Lombardy. Our population showed a median age of 46 years (IQR=24 years) and 95% of distribution was between 15 and 80 years. As shown in Table 1, 68.9% of samples came from northern regions, mainly Lombardy and Piedmont, with a small contribution of Veneto. The remaining 31.1% came from southern regions, mainly Campania (29.5%), with a small contribution (1.6%) from all the other regions.
The overall SARS-CoV-2 IgG prevalence was estimated to be around 11%. Although this data is impacted by the impairment of available sample size and different prescription protocols in different regions, it could be a first indicative results of exposition rate in private laboratory related population. Nevertheless, prevalence ranking was measured in line with cumulative incidence for northern regions involved in this study during the selected time period (2) suggesting a different impact on regional basis. Indeed, the highest prevalence was found in northern regions Lombardy (16.9%), Veneto (20,8%) and Piedmont (7,7%). Interestingly southern regions as Molise (7,5) Campania (7,3) and Sicily (10,7) resulted in comparable prevalence, shown in Figure 1A.
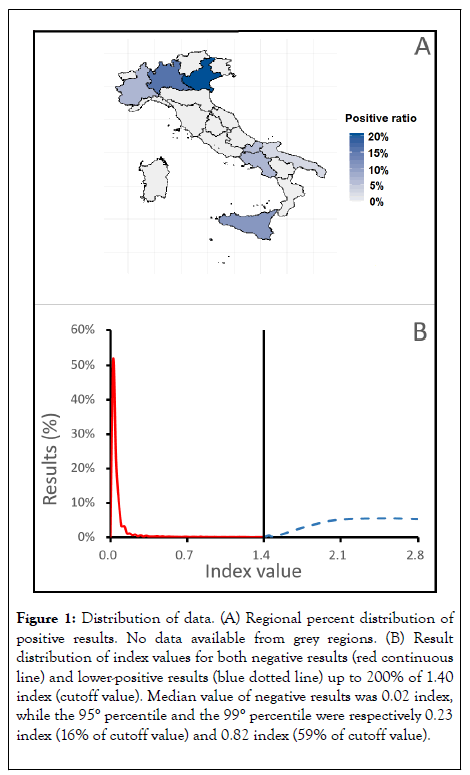
Figure 1: Distribution of data. (A) Regional percent distribution of positive results. No data available from grey regions. (B) Result distribution of index values for both negative results (red continuous line) and lower-positive results (blue dotted line) up to 200% of 1.40 index (cutoff value). Median value of negative results was 0.02 index, while the 95° percentile and the 99° percentile were respectively 0.23 index (16% of cutoff value) and 0.82 index (59% of cutoff value).
To evaluate Abbott Anti-SARS-CoV-2 IgG test response and performance we investigated index signal distribution. Due to sample numerosity only the more representative regions were reported in Table 1. Negative samples where measured showing a distribution far from the positivity cutoff point as shown in Figure 1B.
| Italian Region | Requests (% of total) | Positive (% of total) | Index 1.40 - 2.00 | Index 2.01 - 5.00 | Index>5.00 |
|---|---|---|---|---|---|
| Basilicata | 2 | 0 | ** | ** | ** |
| 0.00% | (*) | ||||
| Calabria | 10 | 0 | ** | ** | ** |
| -0.10% | (*) | ||||
| Campania | 2793 | 203 | 9 | 66 | 111 |
| -29.50% | -7.30% | -5% | -33% | -55% | |
| Lombardy | 3687 | 623 | 37 | 183 | 381 |
| -39.00% | -16.90% | -6% | -29% | -61% | |
| Molise | 80 | 6 | ** | ** | ** |
| -0.90% | -7.50% | ||||
| Piedmont | 2780 | 215 | 16 | 65 | 121 |
| -29.40% | -7.70% | -8% | -30% | -56% | |
| Puglia | 32 | 1 | ** | ** | ** |
| -0.30% | -3.10% | ||||
| Sicily | 28 | 3 | ** | ** | ** |
| -0.30% | -10.70% | ||||
| Veneto | 48 | 10 | ** | ** | ** |
| -0.50% | -20.80% | ||||
| Total | 9460 | 1061 | * % Not calculated if requests<25 | ||
| -100% | -11.20% | ** Not calculated if positives<50 |
Table 1: Collected samples from various regions.
The negative cumulative distribution shows more than 50% results are included in a narrow range<0.10 index and the 99th percentile was calculated at 0.82 index.
The positive samples measured index was clustered in low (1.4-2), medium (2.1-5) and high rate (>5). Results shows relative rates were conserved upon regions. Indeed, results in the low range were measured from 5% to 8%, results in medium range were measured around 30% and most of results (around 60%) were clustered in the higher Index class.
In our experience we hypothesize samples coming from Lombardy, Piedmont and Veneto are representative of a cluster of people coming from municipalities with high incidence that have been subjected to massive testing campaigns.
On the other hand in Campania, the region in which our center is located, and in other southern Italy regions as well the request for IgG testing was more variable and related to different categories such as companies screening employees or private people voluntary screening when coming back from high incidence area or after manifesting some mild symptoms in the near past.
The main limitation of this study is that the results should be considered as representative of an Italian “laboratory afferent population” than of the overall population and data should be considered carefully before making general considerations. Nevertheless, such limitation is the same most hospital have to deal with, therefore our data might be useful. Missing clinical information is also an important drawback of our experience, but this as well is quite common in routine testing.
We consider the selection of population and the IgG test prescribing appropriateness are decisive elements in describing the sero-prevalence of private lab population.
Moreover, since the Abbott Anti-SARS-CoV-2 IgG assay has been demonstrated having a high specificity up to 99,9% [8], at such a consistent prevalence the positive predictive value shall be quite high. This makes the use of this assay extremely reliable for screening purposes ever more considering we have observed a lack of overlapping between the negative and positive results in line with what found elsewhere [8,9].
Also, we found quite interesting that 60% of the positive results falled in a high index range without differences in regions. Infact, even if the ARCHITECTSARS-CoV-2 IgG assay is a qualitative method it has been demonstrated to be associated to sero conversion related IgG Index increase in a time dependent fashion. Several recent studies indicate a different timing and level of IgG expression from symptoms onset in paucisymptomatic vs. severe symptomatic. Nevertheless, few data are available about differences in the same category and, even if further and more specific studies are required, we rely this could be considered as a noteworthy preliminary data.
Citation: Fasano S, Monti A, Cipollaro M, Vita ED, Battista F, Varelli M (2020) SARS CoV 2 IgG Prevalence in Private Laboratories Across Several Italian Regions: The Experience of Varelli Diagnostic Institute. J Clin Chem Lab Med. 3:144. DOI: 10.35248/clinical-chemistry-laboratorymedicine.20.3.144
Received: 22-Jun-2020 Accepted: 06-Jul-2020 Published: 14-Jul-2020
Copyright: © 2020 Fasano S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.