
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2023)Volume 6, Issue 4
Background: Monitoring of therapeutic response, prompt and accurate diagnosis are important for effective treatment and management of children with severe malaria. The serum levels of zinc, copper, iron, magnesium, calcium, inorganic phosphate, sodium, potassium, chloride, creatinine, urea and Uric Acid (UA) were determined using standard methods in 100 children (1 years-10 years) with severe malaria before treatment (day 0), 48 hours of treatment (day 2) and 48 hours after treatment (day 7) according to WHO recommended dosage of Artesunate/ Artemether-Lumefantrine Combination Therapy (AALCT), and 200 clinically healthy children.
Decreased serum Na, and elevated creatinine and urea levels on admission normalised 24 hours after treatment; indicating a fast response. Elevated serum UA concentration showed the highest malaria diagnostic potential (96.5% sensitivity, 83.0% specificity, 7.78% negative predictive value, 91.10% positive predictive value, and 134.61 odds ratio, 0.932 area under the curve at 95% confidence limit).
Conclusion: The results of this study indicated that decreased serum Na, elevated creatinine and urea levels on admission showed fast response to treatment with AALCT in children with severe Falciparum malaria and elevated serum UA level showed a relatively high Falciparum malaria diagnostic potential. Therefore, these can be used as adjuncts to conventional methods.
Severe malaria; Electrolytes; Bio-molecules; Therapeutic response; Diagnostic potential; Children; Artesunate; Artemether-lumenfantrin
Malaria is a mosquito borne disease and it is an endemic disease in Nigeria. Despite several efforts aimed at curbing malaria, its continuous high burden especially in African region and Nigeria in particular, claiming the lives of many especially children is a thing of serious concern [1]. Owing to the continuous increasing incidence of malaria, some shortcomings of current malaria diagnostic and response monitoring methods, coupled with emergence of artemisinin resistant species of Plasmodium there is a need to explore for new method that is more objective, sensitive and fast for malaria diagnosis and monitoring the rate of patient’s recovery. Conventionally, malaria is diagnosed using clinical and laboratory approaches. These are not without their limitations. Thus, WHO in 2006, shifted from presumptive clinical diagnosis of malaria which is based on identification of clinical features such as fever, headache, chills, diarrhoea, vomiting, haemolytic anaemia, jaundice etc [2-5]. Diagnosis based on clinical features has several flaws such as subjectivity, lack of quantification, lack of specificity, variation in diagnosis from different clinicians [6]. Consequently, WHO recommended a laboratory based diagnosis as confirmatory for diagnosis of malaria with microscopy as the gold standard [7,8] and intravenous or intramuscular artesunate as the first line of drug in the treatment of severe malaria. However, microscopy has its limitations both as monitoring and diagnostic tool and these include limitation by the effect of sequestration especially in Falciparum malaria and inter-microscopists variation in reporting the presence of the parasite and parasite density. Use of PCR in malaria diagnosis though compares well with microscopy but due to its ease of contamination and high cost, it is not easily accessible in malaria endemic countries [9]. These flaws may not augur well especially in children with severe malaria among whom the rate of mortality is high and thus needs accurate and prompt monitoring to ascertain patients rate of recovery and response to treatment. This is important because there are individual differences in response to treatment/drugs and to reduce the rate of emergence of artesunate resistant Plasmodium species [10,11].
Artesunate, a semi-synthetic derivative of artemisinin has short half-life (2-3 hours), thus, to prevent development of resistance, artemisinin compounds are combined with one or two longacting antimalarial drugs such as lumefantrine (half-life; 4 days-6 days in Falciparum malaria patients), mefloquine, amodiaquine, or sulfadoxine/pyrimethamine as Artemisinin based Combination Therapy (ACT) [10,11]. Artesunate/artemetherlumefantrine combination therapy is one of the most common combination therapy used in the treatment of severe malaria [12].
Malaria negatively affects host’s physiology, causing derangement in host biochemical processes and parameters especially the electrolytes including Zinc (Zn), Copper (Cu), Iron (Fe), Magnesium (Mg), Calcium (Ca), Inorganic Phosphate (IP), Sodium (Na), Potassium (K) Chloride (Cl) and biomolecules; Creatinine (Cr), Urea (UR) and Uric acid (UA) electrolytes play vital roles in the body and there is need for a balance in their levels for life to be sustained. The trace or microelements-Zn, Cu, Fe, and the macroelements-Mg, Ca, IP, Na, K and Cl are important to maintain health they are part of enzymes, hormones and cells in the body [13-15]. They function in metabolism, nutrition, immunity, homeostasis and regulation of various physiologic functions [16]. The serum level of the bio-molecules; creatinine and urea are important as biomarkers of renal function and UA as marker of nucleic acid catabolism and oxidative stress [17,18].
Sustained imbalance in the levels of these electrolytes and biomolecules can worsen the disease condition and possibly lead to death [13,14]. It has been discovered that the levels of these electrolytes are affected by malaria [15] but the rate of change in their concentrations in response to treatment with AALCT and their malaria diagnostic potential to the best of our knowledge have not been evaluated in Jos University Teaching Hospital (JUTH). This creates the need for the present study.
The diagnostic ability of a parameter or test method for a disease is defined by its diagnostic values. Sensitivity and specificity define the accuracy of a method while the predictive values define the precision. Sensitivity is the probability of the parameter giving a positive result when the patient actually has malaria while specificity is the probability of the parameter giving a negative result when the patient actually does not have malaria [19]. A reliable disease diagnostic parameter or tool should have sensitivity and specificity greater than 90% [20].
Study population
This study was carried out from 28th April 2014 to 15th February, 2016. One hundred children with severe malaria (case children) aged 1 to 10 years, treated at the paediatric wards of Jos University Teaching Hospital (JUTH) Jos and 200 clinically healthy children (1 years-10 years) without malaria attending the hospital for medical check-ups and routine immunization (serving as control) were recruited for the study. Jos located between latitude 80°24'N and longitude 80°32' and 100°38'E is the capital of plateau state in Nigeria.
Children who met the inclusion criteria of the study were recruited consecutively for the study.
• Children aged 1 to 10 years clinically presenting with severe
malaria without any other ailment as diagnosed by the
pediatrician; (severe malaria was diagnosed as the presence of
one or more symptoms of malaria complications e.g anaemia,
respiratory distress, jaundice, etc and detection of malaria
parasite (hyperparasitaemia)/product in the patient’s blood).
• Assent of the child and parent/care-giver’s consent.
• Children microscopically confirmed of hyperparasitaemia.
• Children confirmed by laboratory tests as presenting with only
severe malaria after excluding other disease conditions such as
septicaemia, sickle cell disease, helminthiasis, typhoid,
shigellosis, Glucose-6-Phosphate Dehydrogenase Deficiency
(G6PDD), Human Immune-deficiency Virus (HIV) and
Hepatitis B.
• Children on artesunate/artemether-lumefantrine combination
therapy.
• Children on admission in the hospital using mosquito bednet.
• Children that recovered and were discharged by the 7th day of
admission.
The inclusion criteria for the control children were:
• Children aged 1 years-10 years diagnosed as clinically healthy
by the paediatrician.
• They were without febrile episodes in the past 6 months and
were not on antimalarial drugs for the past 2 weeks or on
paracetamol in the past 24 hours and without any sign of
anaemia or neurological involvement.
• Assent of the child and parent/care-giver consent.
• Children confirmed by laboratory tests as clinically healthy.
• All children enrolled as control were negative for malaria
parasite thick-smear examination (for malaria).
Study design
This was a prospective hospital based case-control study.
Administration of drugs
Children with severe malaria were given 2.4 mg/kg of artesunate intravenously at 0 hours, then 1.2 mg/kg at 12, 24 and 48 hours by the pediatricians and nurses. This was followed by oral administration of artemether-lumefantrine at 5 mg/kg to 24 mg/kg of artemether and 29 mg/kg to 144 mg/kg of lumefantrine for 3 days as fixed dose.
Sample collection and preparation
Five (5 ml) of blood was aseptically collected from the antecubital vein of both case and control subjects using needle and syringe after clinical assessment. In the control, this was done once while in the case children this was done before initiation of treatment on the day of admission (day 0), then 48 hours after initiation of treatment (day 2). Another sample was collected 48 hours after the last dose of the combination therapy i.e 7th day of initiation of treatment.
Two millilitres (2 ml) of the blood was dispensed into EDTA tube for screening tests for exclusion of other abnormalities, malaria parasite and haematological tests. The screening tests carried out include: Haemoglobin genotype for exclusion of sickle cell disease, Glucose-6 Phosphate Dehydrogenase test for exclusion of G6PD deficiency, Hepatitis B surface antigen test for exclusion of viral hepatitis, blood culture for exclusion of septicaemia.
The remaining 3 ml of the blood was dispensed into screw caped plain sample tube for biochemical assays. It was allowed to clot and retract at room temperature for about 20 minutes. The serum was separated after centrifuging at 3000 rpm for 5 minutes in a clinical bench top centrifuge (MSE minor England) using Pasteur pipette and divided into three different aliquots into pre-cleaned, dried, metal and steroid free cryo-vials. They were stored at -20℃ for analysis of biochemical parameters. Serum HIV screening was carried out immediately on the serum sample.
Stool sample was collected into transparent stool containers from both case and control children for exclusion of helminthiasis and pathological enteric bacterial infection using stool microscopy and culture tests.
Malaria parasite test
Malaria parasite test was by microscopy method using Giemsa stained duplicate thick and thin slides. The duplicate slides were blindly read by an experienced clinical/medical laboratory scientist who is involved in the study. This served as the internal quality control. The slides were also read by another expert microscopist who is not otherwise involved in the study (independent reader) this served as the external quality control. The degree of variation in the results was determined and subjected to statistical analysis at 95% confidence limit to test for significance.
A film was reported as malaria parasite not seen (negative) if at the end of examining about 100 fields no Malaria Parasite (MP) was seen. However, if MP was seen, then the specie was ascertained using the giemsa stained thin film and reported accordingly. The parasite density count (parasitaemia) was carried out using the number of parasites/μl of blood method (thick film) using respective patient’s total white blood cell count as expressed below:
Parasite density (× 103 parasites/μl)=(N × Paitent’s own total WBC count/ μl)/Leucocytes count (200)
Where;
N=number of parasites counted in 200 leucocytes count.
Determination of serum levels of some electrolytes and biomolecules
Serum zinc ion concentration was quantified colorimetrically using 2-(5-Brom-2-Pyidylazo)-5-(N-propyl-N-Sulfoproplamino)- Phenol (5-Br-PAPS) method as described by Johnson and Eliasson. Serum copper ion concentration was determined colorimetrically using 4-(3,5-dibromo-2-pyridylazo) N-ethyl-Nsulfopropylaniline (PAESA) method as described by Abe, et al. Serum iron ion concentration was quantified colorimetrically using guanidine hydrochloride and nitro-PAPS (nitro-propyl-Nsulfoproplamino) phenol as described by Makino, et al. Serum magnesium ion concentration was quantified colorimetrically using xylidyl blue method as described by Mann and Yoe. Serum calcium ion concentration was quantified colorimetrically using modified O-cresolphtalein complex method as described by Cohen and Sideman. Phosphomolybdate method was used colorimetically to quantify serum inorganic phosphate concentration as described by Rubino, et al.
Serum sodium ion level was quantified colorimetrically by uranyl acetate method as described by Trinder. Serum potassium ion level was quantified colorimetrically by turbidometric method using sodium hydroxide and tetraphenylborate-sodium as described by Bladh and Gedda.
Serum chloride ion concentration was quantified colorimetrically using modified thiocyanate method as described by Charles and Alberto. Serum uric acid concentration was determined colorimetricaly using the method described by Lopera-Mesa, et al. Serum creatinine concentration was quantified colorimetrically using picric acid method (Jaffe’s reaction) as described by Amin, et al. Urea concentration was quantified colorimetrically using the modified Berthelot method as described by Amin, et al. Tests were run in duplicate tubes. All the reagents used for this study were of analytical grade and were prepared in all glass distilled water.
Statistical analysis
The results were presented as mean ± standard error of mean. Duncan’s multiple range tests according to montgomery was used for the analysis involving determination of therapeutic response. P-value of less than 0.05% at 95% confidence limit was considered statistically significant in all analysis.
The responses of the parameters were categorised as: Fastest response (those that their mean levels increased or decreased steadily such that there was no significant difference between their day 2 through day 7 levels when compared with the mean control values; fast response (those that increased or decreased steadily and their mean levels by day 7 were not significantly different with the control); slow response (those that increased or decreased steadily and their mean levels by day 7 were significantly different with control), biphasic response (those that did not show a steady rise or decrease in their mean level from admission to the day of discharge; no-response (those that steadily showed non-significant rise or decrease in their mean level from day of admission to discharge).
To determine the diagnostic potential of the parameters, linear regression was used to establish parameters that are significant predictors of malaria. Reference range was defined as mean ± 2 SD values of the parameters in certified clinically healthy control children.
A 2 × 2 contingency table with the gold standard (microscopy) occupying the column and the potential new test methods (electrolytes and biomolecules) occupying the row was used by cross-matching the number of the results of the potential new methods with that of the gold standard to determine the numbers of the results of the potential new methods that are: True Positives (TP), False Positives (FP), True Negatives (TN) and False Negatives (FN).
Sensitivity, specificity, predictive values and odds ratios of the potential new diagnostic methods were calculated using the formula below:
Sensitivity (%)=(TP/(TP+FN)) × 100 Specificity (%)=(TN/((TN+FP)) × 100 Negative predictive value (%)=(FP/(FN+TN)) × 100 Positive predictive value (%)=(TP/(TP+FP)) × 100 Odds ratio=TP/FP÷FN/TN
The Receiver Operational Characteristic (ROC) curve analysis was used to revalidate the cut-off values, further confirm the diagnostic values and determine the area under the curve of the tests.
Serum concentrations and responses of some trace elements in case and control children with severe malaria before, during and after treatment
The serum concentrations and responses of trace minerals Zn, Cu and Fe are shown in Table 1. Serum Zn and Fe levels were significantly (p<0.05) lower before commencement of treatment in children with malaria compared with the control. The concentrations increased steadily and significantly (p<0.05) during and after treatment (day 2 and day 7 respectively) and were significantly (p<0.05) higher than the controls with Fe being significantly (p<0.05) lower 48 hours after treatment (day 7) compared with the controls. This indicates that the trace elements showed slow response to treatment (Table 1). Serum Cu concentration was significantly higher (p<0.05) in children with malaria before treatment compared with the control. However, treatment with AALCT significantly (p<0.05) decreased the level 48 hrs of treatment with slight nonsignificant (p>0.05) rise 48 hours after treatment. This denotes a biphasic response.
| Trace-elements | Cases | |||
|---|---|---|---|---|
| Control | Day 0 | Day 2 | Day 7 | |
| Mean ± S.E.M | Mean ± S.E.M | Mean ± S.E.M | Mean ± S.E.M | |
| Zinc (µg/dl) | 90.92 ± 2.01a | 50.74 ± 1.87b | 119.34 ± 0.81c | 141.72 ± 0.77d |
| Copper (µg/dl) | 128.85 ± 7.35a | 270.38 ± 5.93c | 165.04 ± 6.17b | 168.26 ± 4.25b |
| Iron (µmol/l) | 6.10 ± 0.05a | 4.28 ± 0.09d | 5.38 ± 0.07c | 5.83 ± 0.05b |
Note: Each value is a mean of n determinations ± S.E.M. (n is 100 for children with malaria and 200 for control children). Values with different superscripts along the same row are significantly different (p<0.05).
Day 0=before treatment; Day 2=48 hours of treatment with artesunate; Day 7=48 hours after treatment with artesunate/artemether-lumefantrine combination therapy.
Table 1: Serum concentrations and responses of some trace (micro) elements in case and control children with severe malaria before, during and after treatment.
Serum concentrations and responses of some macroelements in children before, during and after treatment with artesunate/artemether-lumefantrine combination therapy
Serum Mg level was significantly (p<0.05) lower before commencement of treatment in children with malaria compared with the control. The concentration increased steadily and significantly (p<0.05) during and after treatment (day 2 and day 7 respectively) and was significantly (p<0.05) higher than the controls. The Table 2 showed that there was no significant difference (p>0.05) in serum Ca levels in children with malaria before treatment, 48 hours after start of treatment (day 2) and 48 hours after treatment with artesunate/artemetherlumefantrine combination therapy (day 7) compared with the controls. This denotes no-response. Serum IP was significantly (p<0.05) lower in children with malaria before, during and after treatment compared with the controls. Serum Na level was significantly (p<0.05) lower in children with malaria before treatment (day 0) and during treatment (day 2), but normalised 48 hours after treatment (day 7) indicating a fast response. Serum K level was significantly (p<0.05) higher in children with malaria before treatment and 48 hours after the start of treatment (day 2) but decreased significantly (p<0.05) after treatment (day 7) with artesunate/artemether-lumefantrine combination therapy compared with the controls. There was no significant (p>0.05) difference in serum chloride concentrations in the case children before and during treatment compared with control, it increased significantly (p<0.05) after treatment.
| Macro-elements | Cases | |||
|---|---|---|---|---|
| Controls | Day 0 | Day 2 | Day 7 | |
| Mean ± S.E.M | Mean ± S.E.M | Mean ± S.E.M | Mean ± S.E.M | |
| Magnesium (mg/dl) | 2.13 ± 0.05a | 1.15 ± 0.06c | 1.80 ± 0.05b | 2.39 ±0.11d |
| Calcium (mg/dl) | 10.28 ± 10.12ab | 10.41 ± 0.15b | 10.15 ± 0.13ab | 9.92 ± 0.09a |
| IP (mg/dl) | 4.32 ± 0.05a | 3.96 ± 0.16b | 3.52 ± .08c | 3.87 ± .09b |
| Sodium (mmol/l) | 1 38.03 ± 1.25a | 118.12 ± 2.02c | 131.15 ± 1.59b | 137.28 ± 0.36a |
| Potassium (mmol/l) | 4.21 ± 0.04a | 4.57 ± 0.09c | 4.79 ± 0.02d | 3.85 ± 0.04b |
| Chloride (mmol/l) | 93.01 ± 0.48a | 91.48 ± 0.87a | 92.47 ± 0.25a | 98.77 ± 0.50b |
Note: Each value is a mean of n determinations ± S.E.M. (n is 100 for children with malaria and 200 for control children). Values with different superscripts along the same row are significantly different (p<0.05).
Day 0=before treatment; Day 2=48 hours of treatment with artesunate; Day 7=48 hours after treatment with artesunate/artemether-lumefantrine combination therapy.
Table 2: Serum concentrations and responses of some macroelements in children before, during and after treatment with artesunate/artemether-lumefantrine combination therapy.
Serum concentrations and responses of some bio-molecules in children with severe Falciparum malaria before, during and after treatment with artesunate/artemether-lumefantrine combination therapy
The serum concentrations and responses of some biomolecules in children with severe Falciparum malaria before, during and after treatment with artesunate/artemether-lumefantrine combination therapy are shown in Table 3. Serum creatinine, urea and UA concentrations were significantly (p<0.05) higher in children with malaria before the start of treatment compared with the controls. However, treatment with artesunate/ artemether-lumefantrine combination therapy steadily and significantly (p<0.05) lowered the concentrations during and after treatment compared with controls. Serum creatinine and urea levels normalised 48 hours after treatment (day 7); indicating a fast response while serum UA level was significantly (p<0.05) higher 48 hours after treatment compared with controls; indicating a slow response (Table 3).
| Bio-molecules | Cases | |||
|---|---|---|---|---|
| Control | Day 0 | Day 2 | Day 7 | |
| Mean ± S.E.M | Mean ± S.E.M | Mean ± S.E.M | Mean ± S.E.M | |
| Creatinine (µmol/l) | 5.48 ± 0.24a | 31.29 ± 2.73c | 10.26 ± 0.24b | 7.98 ± 0.43ab |
| Urea (mmol/l) | 1.32 ± .06a | 4.53 ± 0.34c | 1.94 ± 0.07b | 1.68 ± 0.08ab |
| Uric acid (mg/dl) | 4.82 ± 0.12a | 12.73 ± 0.48d | 11.95 ± 0.12c | 5.89 ± 0.10b |
Note: Each value is a mean of n determinations ± S.E.M. (n is 100 for children with malaria and 200 for control children). Values with different superscripts along the same row are significantly different (p<0.05).
Day 0=before treatment; Day 2=48 hours of treatment with artesunate; Day 7=48 hours after treatment with artesunate/artemether-lumefantrine combination therapy.
Each value is a mean of n determinations ± S.E.M. (n is 100 for children with malaria and 200 for control children). Values with different superscripts along the same row are significantly different (p<0.05).
Day 0=before treatment; Day 2=48 hours of treatment with artesunate; Day 7=48 hours after treatment with artesunate/artemether-lumefantrine combination therapy.
Table 3: Serum concentrations and responses of some biomolecules in children with severe Falciparum malaria before, during and after treatment with artesunate/artemether-lumefantrine combination therapy.
Serum electrolyte and biomolecule predictors of malaria in children
The result of linear regression analysis is shown in Table 4. It showed that Zn, Fe, Na, K, creatinine, urea and uric acid were significant (p<0.05) predictors of malaria.
| Model | Unstandardized coefficients | Standardized coefficients Beta | t | Sig. | 95.0% confidence interval for B | ||
|---|---|---|---|---|---|---|---|
| B | Std. error | Lower bound | Upper bound | ||||
| (Constant) | -0.108 | 0.418 | -0.259 | 0.796 | -0.932 | 0.715 | |
| Zn | -0.004 | 0.001 | -0.325 | -4.347 | 0 | -0.005 | -0.002 |
| Cu | 7.04E-05 | 0 | 0.011 | 0.205 | 0.838 | -0.001 | 0.001 |
| Fe | -0.082 | 0.028 | -0.172 | -2.946 | 0.003 | -0.136 | -0.027 |
| Mg | -0.047 | 0.024 | -0.091 | -1.934 | 0.054 | -0.095 | 0.001 |
| Ca | 0.061 | 0.018 | 0.168 | 3.439 | 0.001 | 0.026 | 0.095 |
| IP | -0.062 | 0.019 | -0.151 | -3.232 | 0.001 | -0.099 | -0.024 |
| Na | 0.006 | 0.002 | 0.228 | 3.973 | 0 | 0.003 | 0.01 |
| K | 0.165 | 0.038 | 0.252 | 4.4 | 0 | 0.091 | 0.239 |
| Cl | -0.006 | 0.004 | -0.083 | -1.422 | 0.156 | -0.014 | 0.002 |
| Creatinine | -0.003 | 0.002 | -0.108 | -1.52 | 0.13 | -0.006 | 0.001 |
| Urea | -0.005 | 0.013 | -0.026 | -0.378 | 0.705 | -0.032 | 0.021 |
| UricAcid | 0.033 | 0.007 | 0.299 | 4.507 | 0 | 0.018 | 0.047 |
Table 4: Serum electrolyte and bio-molecule predictors of malaria in children.
Normal range of serum electrolyte and biomolecule predictors of malaria in children and cut-off values for definition of malaria positives
The normal serum ranges which is mean ± 2 standard deviations for Zn, Fe, Ca, IP, Na, K, and UA in clinically certified healthy children (control) were determined (Table 5). For the parameters (Ca, K and UA), which had their values increased due to malaria before treatment, the cut-off values for malaria positive were defined as values greater than the normal ranges (means plus 2 standard deviations of the control values) and those within the normal range were termed malaria negative. For the parameters (Zn, Fe, IP, Na) which had their values decreased due to malaria before treatment, the cut-off values for malaria positive was defined as values lower than the normal ranges (mean minus 2 standard deviations of the control values) while those within the normal ranges were termed malaria negative.
| Potential new test | Children with severe malaria (day 0) | Children without malaria control children | Reference range control children | Cut-off value for malaria positive |
|---|---|---|---|---|
| Mean ± SD (n=100) | Mean ± SD (n=200) | Mean ± 2 SD (n=200) | ||
| Zinc (µg/dl) | 50.73 ± 18.700 | 90.92 ± 29.32 | 32.28-149.56 | <32.28 |
| Iron (µmol/l) | 4.27 ± 0.913 | 6.10 ± 0.66 | 4.78-7.42 | <4.78 |
| Calcium (mg/dl) | 10.40 ± 1.592 | 10.29 ± 1.65 | 6.99-13.59 | >13.59 |
| IP (mg/dl) | 3.96 ± 1.580 | 4.32 ± 0.82 | 2.68-5.96 | <2.68 |
| Sodium (mmol/l) | 118.12 ± 20.247 | 138.03 ± 17.77 | 102.49-173.54 | <102.49 |
| K (mmol/l | 4.56 ± 0.925 | 4.21 ± 0.55 | 3.11-5.31 | >5.31 |
| Uric acid mg/dl | 12.73 ± 4.760 | 4.82 ± 1.75 | 1.32-8.32 | >8.32 |
Table 5: Reference ranges and cut-off values of serum electrolytes and bio-molecules (predictors of malaria) for diagnosis of Falciparum malaria in children.
Malaria diagnostic potential of serum electrolytes and biomolecules that are predictors of malaria in children with severe Falciparum malaria treated with artesunate/artemetherlumefantrine combination therapy
Serum UA level, above the cut-off value of >8.32 mg/dl, indicative of malaria, had the best diagnostic values (96.5% sensitivity, 83.0% specificity, 7.78% Negative Predictive Value (NPV), 91.10% Positive Predictive Value (PPV), and 134.61 Odds Ratio (OR) for diagnosis of Falciparum malaria in children compared to other parameters (Table 6). The other parameters had relatively high specificity with low sensitivity, indicating that they are not good malaria diagnostic indices.
| Potential new test method | TN (N) | FP (N) | Total (N) | TP (N) | FN (N) | Total | Sen. (%) | Spec. (%) | NPV (%) | PPV (%) | OR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zinc (<32.28 ug/dl) | 100 | 0 | 100 | 21 | 179 | 200 | 10.5 | 100 | 64.12 | 100 | 11.73 |
| Iron (<4.78 umol/l) | 98 | 2 | 100 | 81 | 119 | 200 | 40.5 | 98 | 54.84 | 97.57 | 33.32 |
| Calcium (>13.59 mg/dl) | 100 | 0 | 100 | 13 | 187 | 200 | 6.5 | 100 | 65.16 | 100 | 6.95 |
| IP (<2.68 mg/dl) | 95 | 5 | 100 | 30 | 170 | 200 | 15 | 95 | 64.15 | 85.71 | 3.34 |
| Na (< 102.49 mmol/l) | 100 | 0 | 100 | 34 | 166 | 200 | 17 | 100 | 62.41 | 100 | 20.48 |
| K (>5.31 mmol/l) | 100 | 0 | 100 | 27 | 173 | 200 | 13.5 | 100 | 63.37 | 100 | 15.61 |
| Uric acid (>8.32 mg/dl) | 83 | 17 | 100 | 193 | 7 | 200 | 96.5 | 83 | 7.78 | 91.1 | 134.61 |
Note: TN: True Negative; FP: False Positive; TP: True Positive; FN: False Negative; Sen: Sensitivity; Spec.: Specificity; NPV: Negative Predictive Value; PPV: Positive Predictive Value; OR: Odds Ratio
Table 6: Diagnostic values of serum electrolyte and bio-molecule predictors of Falciparum malaria in children.
Determining the malaria diagnostic potential of serum electrolytes and biomolecules that are predictors of malaria using receiver operational characteristic curve
We further confirmed the diagnostic values of the electrolytes and biomolecules that were significant predictors of malaria using the Receiver Operational Characteristic (ROC) curve in Figure 1. The ROC curve shows the sensitivity representing the presence of malaria (true positive) using electrolytes and biomolecules that were significant predictors of malaria (potential new test methods) against the false positive (1- specificity). Presence or absence of malaria is the external criterion in this study. Parameters with curves above the middiagonal reference line and tended towards left had stronger evidence for a true positive result (high accuracy in diagnosing the presence of malaria) while parameters with curves below the line and tended towards right had stronger evidence for a false positive result (low accuracy in diagnosing the presence of malaria). Serum UA had its curve above the mid-diagonal reference line and tended towards left, indicating high sensitivity in malaria diagnosis while serum Zn, Fe, Ca, IP and Na had curves below the line and tended towards right, indicating low accuracy in malaria diagnosis (Figure 1). These were confirmed by the areas under the curve occupied by the various parameters (UA=0.932, K=0.860, Ca=0.537, IP=0.338, Fe=0.197, Na=0.120, Zn=0.046 at 95% confidence interval) (Table 7).
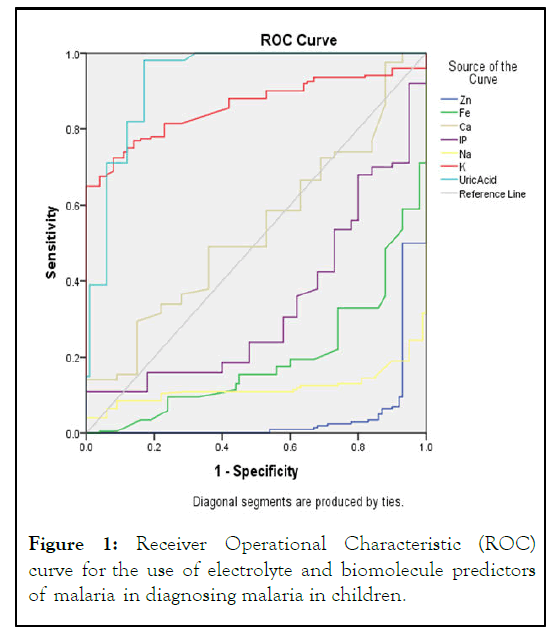
Figure 1: Receiver Operational Characteristic (ROC) curve for the use of electrolyte and biomolecule predictors of malaria in diagnosing malaria in children.
| Test result variable (s) | Area | Std. error | Asymptotic sig. | Asymptotic 95% confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Zn (µg/dl) | 0.046 | 0.014 | 0 | 0.02 | 0.073 |
| Fe (µg/dl) | 0.194 | 0.025 | 0 | 0.145 | 0.244 |
| Ca (mg/dl) | 0.537 | 0.034 | 0.296 | 0.47 | 0.604 |
| IP (mg/dl) | 0.338 | 0.033 | 0 | 0.274 | 0.402 |
| Na (mmol/l) | 0.12 | 0.021 | 0 | 0.079 | 0.161 |
| K (mmol/l) | 0.86 | 0.021 | 0 | 0.819 | 0.901 |
| Uric acid (mg/dl) | 0.932 | 0.017 | 0 | 0.898 | 0.966 |
Table 7: Area under the curve for diagnostic potentials of predictors of Falciparum malaria.
For proper treatment and management of children with severe malaria, there is the indispensable need for proper and timely diagnosis and monitor of patient’s rate of recovery. Potentials of electrolytes and bio-molecules as therapeutic monitoring and diagnostic tools were explored in this study. The study has shown that serum levels of Na, creatinine and urea showed fast response (parameters’ serum levels returning to normalcy 24 hours after treatment) to treatment with AALCT in children with severe Falciparum malaria and thus, could be used to monitor the rate of recovery in such patients. None of the parameters studied showed a fastest response (parameters’ serum levels returning to normalcy 24 hours after initiation of treatment). Elevated serum UA level showed a relatively high Falciparum malaria diagnostic potential.
The low serum level of Zn, Fe, Mg, IP and Na, observed in this study before the start of treatment is in agreement with the report of Maitland, et al., and Jimoh, et al. However, Rani, et al., reported low serum K level in children with malaria. Our finding may be due to transcellular shift in the levels of these elements in order to offset some of the effects of plasmodium, this as was also suggested by some other authors. It may also be due to the parasite using these minerals from the host for its metabolic functions. Low serum levels of Fe and Mg may also be due to hemolysis.
Restoring and maintaining the levels of these parameters within the normal range is crucial for recovery and sustenance of life. With the administration of artesunate/artemether-lumefantrine combination therapy the decreased concentrations of serum Zn, Fe, Mg showed a slow response to treatment. The slow response of these elements may be because these elements are required only in trace amount in the body, coupled with their increased utilization in the course of recovery so as to offset the effects of their decline due to malaria. The oxidative effect of artemisinin therapy on these elements may also contribute to their slow response as most of them function in redox reactions. This implies that the serum levels of these elements, though, are affected by malaria are poor therapeutic response monitoring parameters. So, though there is need to measure their concentration in children with malaria due to their physiological roles in the body and detrimental effects of derangement in their levels, they should not be used as therapeutic response monitoring parameter (s) for monitoring patient’s rate of recovery because they showed slow response.
Serum sodium concentration and osmolality are closely regulated by water homeostasis which in turn is affected by diarrhoea, vomiting, fever which is some of the clinical features of malaria observed among our case children. With the administration of artesunate/ artemether-lumefantrine combination therapy the decreased concentrations of serum Na on admission was normalised 24 hours after treatment with AALCT. This depicts a fast response. This implies that serum concentration of Na can be used for monitoring therapeutic response in children with severe Falciparum malaria. The fast response noted in elevated serum Na concentration may be due to active membrane transport mechanism involving Na. This may be because of increased membrane permeability to Na into extracellular fluid to offset the effect of Na imbalance. This follows the administration of normal saline in children with severe malaria. Also, dissolution of artesunate in sodium bicarbonate to form sodium artesunate before administration, may also, have caused a fast rise in serum Na level following treatment with artesunate.
Elevated serum Cu and K concentrations in children with malaria before the initiation of treatment observed in this study, is in concordance with that reported by Maitland, et al., and Jimoh, et al. Elevated serum copper in malaria infection may be due to an attempt of the body to respond to malaria parasite infection as immune response or the inhibition of zinc absorption from the intestine. K being a majorly intracellular cation, its high serum K level may be due to lysis of RBC in malaria infection leading to release of K into circulation thereby elevating the serum level before the initiation of treatment.
Also, elevated serum Cu and K levels on admission showed biphasic and slow response respectively on treatment with AALCT. Therefore they are unfit for monitoring therapeutic response. However, bearing in mind the detrimental effects of low plasma K on the heart, the significantly decreased level of K after treatment with AALCT (day 7) compare with the controls calls for attention on the need to monitor the level of this mineral after the course of treatment with AALCT in children.
Severe malaria also affected the renal functions and caused increased oxidation of nucleic acid as evidenced by significantly (p<0.05) elevated serum creatinine, UR (markers of renal function) and UA (marker of oxidation) levels obtained before treatment in the case children as compared with the control (Table 3). This compares positively with the report of some other authors. The elevated serum uric acid may be due to increased oxidation in malaria that led to increased damage of nucleic acids. This agrees with the report of Gowda, et al., and Lopera- Mesa, et al. Serum creatinine and UR levels showed a fast response to treatment. This implies that serum creatinine, and UR levels are markers of fast response to treatment. Thus, assessment of their level is important in managing and monitoring response to treatment in children with severe Falciparum malaria treated with AALCT.
For proper treatment and management of malaria, accurate diagnosis is important. Due to some of the shortcomings of conventional methods of malaria diagnosis, there is a need to explore for a more objective, sensitive and accurate method of malaria diagnosis. The serum levels of the electrolytes Zn, Fe, Ca, IP, Na which are significant predictors of malaria showed less than 17% sensitivity with only Fe having 40.5%sensitivity. However, all the electrolytes had >90% specificity but low odds ratios. This implies that these electrolytes lack good malaria diagnostic potential even though alteration in their normal values was associated with malaria severity. Thus, considering the fact that other tests were done to rule out for other infections such as bacterial and viral that could produce similar biochemical changes, the poor malaria diagnostic potential of these parameters could then be due to the influence of other factors such as diet, absorption, excretion, nutritional status, oxidative effect of artemisinins individual difference in response to artemisinin treatment, level of parasitaemia and state of host immunity. Moreover, it has been reported that artemisinins act by inhibiting a calcium ATPase (PfATP6) leading to take up of free calcium thereby elevating cystolic free calcium, thus resulting in increased false negative results (low sensitivity) in using serum Ca>13.59 mg/dl (hypercalcaemia) or ≤ 6.99 mg/dl (hypocalcaemia) as respectively indicative of presence or absence of malaria. Also, low sensitivity of hypozincaemia (Zn<32.28 umol/dl) may be attributed to the fact that artemisinin preparations contain trace minerals such as zinc, copper, Fe etc. thereby, falsely elevating the serum level of this parameter and resulting in increased false negative results (low sensitivity). Likewise, low sensitivity (41.5%) of hypoferrousaemia (Fe<4.78 umol/l) could be attributed to the haemolytic effect of artemisinins and presence of these trace minerals in the drug thus, falsely elevating the value of Fe. Also, low sensitivity of hyponatreamia (Na<102.49 mmol/l) may be because of dissolution of artesunate in sodium bicarbonate to form sodium artesunate before administration, thus falsely elevating the serum level of this mineral.
Conversely, the high sensitivity, specificity and precision of hyperuricaemia (uric acid>8.32 mg/dl) could be because of the oxidative effects of malaria and artemesinins with the fact that it has been reported that there is no binding between DNA and artemsinins. Uric acid is a marker of nucleic acid breakdown. High sensitivity of hyperuriceamia was further buttressed using the ROC curve. Serum uric acid level at cutoff value >8.32 mg/dl (hyperuricaemia)as indicative of malaria showed sensitivity 96.5%, specificity 83%, NPV 18.89%, PPV 91.10%, OR 141.88; AUC=0.932 at 95% C.I). This implies that using elevated serum UA level of >8.32 mg/dl as indicative of malaria in children will result in use of antimalarial drugs in 17% of malaria negative cases (specificity=83%) but will only result in less than 5% of true malaria cases not treated (sensitivity=96.5%). It also indicates that these children were 134.61 times more likely to have malaria than those with normal serum UA concentration (odds ratio=134.61 at 95% C.I) and a child who tests positive by UA level method has 91.10% likelihood of having malaria (PPV=91.10%) and 7.78% likelihood of not having malaria (NPV=7.78%). This agrees with the high prevalence of P. falciparum malaria in this region of the world.
From our study, microscopy remains a very good gold standard for laboratory diagnosis of malaria and elevated serum UA level >8.32 mg/dl as indicative of Plasmodium falciparum malaria compares favourably with it.
Thus, using serum levels of Na, creatinine and UR as adjuncts to conventional methods of monitoring therapeutic response has an advantage of giving a fast response as indicative of positive response to treatment in children with severe Falciparum malaria. Proper application of this knowledge can help improve on correct assessment of patient's response to treatment, thereby helping in proper management of these patients thus, helping to reduce morbidity and mortality due to severe Falciparum malaria in children.
The results of this study indicated that decreased serum Na, and elevated creatinine and urea admission levels showed fast response to treatment with AALCT in children with severe Falciparum malaria and thus, could be used in conjunction with conventional methods to monitor the rate of recovery in such patients. The result also showed that elevated serum UA level showed a relatively high Falciparum malaria diagnostic potential and could be helpful as an adjunct to microscopy.
This study was carried out in line with the ethics guiding research undertakings on human subjects as approved by the ethical committees of university of Ilorin and Jos university teaching hospital. Informed consent to collect ward’s samples and publish the result of the finding of this research was sought and obtained from the children’s parents or care givers before enrolment, after due explanation of the aims and procedures of the project.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Okoli CA, Igunnu A, Oguche S, Malomo SO (2023) Response Monitoring and Diagnostic Potentials of Some Serum Electrolytes and Bio-Molecules in Children with Severe Falciparum Malaria Treated with Artesunate/Artemether-Lumefantrine Combination Therapy. J Clin Chem Lab Med. 6:271.
Received: 15-Mar-2023, Manuscript No. JCCLM-23-22186; Editor assigned: 17-Mar-2023, Pre QC No. JCCLM-23-22186 (PQ); Reviewed: 31-Mar-2023, QC No. JCCLM-23-22186; Revised: 18-May-2023, Manuscript No. JCCLM-23-22186 (R); Published: 25-May-2023 , DOI: 10.35248/2736-6588.23.6.271
Copyright: © 2023 Okoli CA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.