Transcriptomics: Open Access
Open Access
ISSN: 2329-8936
ISSN: 2329-8936
Research Article - (2015) Volume 3, Issue 2
Although soil bacterial communities are one of the important biotic components that influence decomposition and nutrient mineralization in the terrestrial ecosystems, factors driving this biotic community in the broadleaved forest stands of Meghalaya are not well studied. The present study examined the importance of physico-chemical properties in driving soil bacterial communities in the broadleaved forest stands of Meghalaya differing in altitudes. Soils were collected at two different (0-10 cm and 10-20 cm) depths monthly for a period of two years. Results showed that bacterial CFU was higher in the high altitude forest stand at 0-10 cm depth as compared to the low altitude forest stand at 10-20 cm depth. It also showed significant positive correlation with organic carbon and total nitrogen in both the two forest stands indicating that these two constitute the major driving factors of bacterial communities in the broad leaved forest stand of Meghalaya.
<Keywords: Bacterial population; Physico-chemical properties; Broad leaved forest
Microbial diversity is an unseen national as well as international resource that deserves greater attention than it has been receiving. It encompasses the spectrum of variability among all types of microorganisms in the natural world and as altered by human intervention. Microbial diversity studies are important in order to understand the microbial ecology in soil and other ecosystems Atlas [1]. It plays an important role in both natural and agro-ecosystems. The diversity of plants and animals in forests and agro-ecosystems receives a great deal of scientific attention, whereas, the diversity of microorganisms is often ignored. Therefore, much more needs to be done to understand the role of microorganisms and inventory of their diversity and to find ways to exploit them beneficially. Of this, soil bacteria are one of the important biotic components that influence decomposition and nutrient mineralization in the terrestrial ecosystems Bardgett [2]. Although studies on examining the factors that influence the soil microbial communities in various ecosystems such as agricultural fields, grasslands and forests are substantial Hossain and Sugiyama [3]; Nusslein and Tiedje [4], relatively less information is available on the relationship between soil properties and microbial communities in broadleaved forest soils that are characteristically different from other terrestrial ecosystems. It is important to study microbial diversity not only for basic scientific research, but also to understand the link between diversity and community structure and function. Although plant and soil microbial communities are closely associated, the relationships between plant diversity and microbial process have been little explored Broughton and Gross [5]. Our knowledge of plant-microbe interactions is increasing, but the complexity of interacting biological, chemical and physical factors remain to be understood. Despite all attempts to measure fluxes and gross microbial pools, the soil and its micro biota still remain a black box since, most soil microorganisms are still unknown Crecchio et al. [6]. Evaluating soil physico-chemical characteristics provided useful information on soil microbial activity. It is necessary to study the interrelationship between the physical, chemical properties of soil in order to see the microbial activities Meliani et al. [7]. However, although some reports on the relationship between soil microbial communities and soil properties are available, relatively less attention has been paid on the broadleaved forest stands of Meghalaya. The objectives of the present study were to study the bacterial CFU and to examine the relative importance of the various physico‐chemical properties in driving bacterial CFU in the broadleaved forest soils.
Study site description and soil sampling
For the present investigation, two broad leaved forest stands at different altitudes of Meghalaya were selected. The study sites selected were Upper Shillong at a higher altitude and Mawkyrdep at a lower altitude. The forest stand at a higher altitude is situated at 1861 m above sea level and lies at 25⁰ 32′ 17.1′′ N latitude and 91⁰ 51′ 03.0′′ E longitude. The other forest stand at a lower altitude is situated at 889 m above sea level and lies at 25⁰ 41′ 10.5′′ N latitude and 92⁰ 03′ 44.90′′ longitudes. Soil samplings were done at two different depths 0-10 cm and 10-20 cm for a period of two years i.e., 2009 and 2010. The soil samples were collected aseptically from 5 different places at each study site and were thoroughly mixed to make a composite sample. This was done to minimize local variation in the bacterial population.
Bacteriological analysis
For the isolation of bacteria, serial dilution method given by Johnson and Curl, was followed using Nutrient Agar medium (Difco manual). Three replicates were maintained for each sample. Inoculated Petri plates were then incubated upside down at 30 ± 1℃ for 24 hours in a BOD incubator. The number of bacterial colonies was counted and the Colony Forming Unit (CFU) was calculated based on dry weight basis.
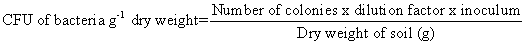
Analysis of physico-chemical properties
Soil temperature was noted at the time of sampling using a digital soil thermometer. The soils were screen to remove plants roots, rocks and macrofauna. Moisture content was determined by oven dry basis. pH was read using a digital pH meter. Dried and sieved soil through 2 mm mesh was used for the remaining analysis. Organic carbon was estimated following the method given by Anderson and Ingram [8]. Total nitrogen was estimated following Kjeldahl distillation and titration method given by Jackson [9]. Available phosphorus was estimated by molybdenum blue method given by Allen [10]. Exchangeable potassium was determined by flame photometer method given by Jackson [9].
Bacterial CFU exhibited monthly variations at the two different soil depths (0-10 cm and 10-20 cm) at the two forest stands during the study periods i.e., 2009 and 2010. The soil at the high altitude forest stand harboured higher bacterial CFU as compared to the low altitude forest stand. Higher bacterial CFU was recorded at 0-10 cm depth than at 10-20 cm depth at both the forest stands. With increase in depth, there was decrease in bacterial CFU. In the first year of the study period at the high altitude forest stand at 0-10 cm, maximum bacterial CFU was recorded in the month of April and the minimum was recorded in the month of December, whereas, at the low altitude forest stand at 0-10 cm depth, maximum bacterial CFU was recorded in the month of April and the minimum was recorded in the month of October. At 10-20 cm depth at both the forest stands, maximum bacterial CFU was recorded in the month of May and the minimum was recorded in the month of October. In the second year of the study period at the high altitude forest stand at both the depths, maximum bacterial CFU was recorded in the month of July and the minimum was recorded in the month of October, whereas, at the low altitude forest stand at both the depths, maximum bacterial CFU was also recorded in the month of July and the minimum was recorded in the month of November (Figure 1). In the first year of the study period at the high altitude forest stand, bacterial CFU ranged between 20.00-60.00 at 0-10 cm depth. At 10-20 cm depth, it ranged between 20.00-50.00, whereas, at 0-10 cm depth at the low altitude forest stand, it ranged between 12.00-35.00. At 10-20 cm depth, it ranged between 10.00-20.00. In the second year of the study period at the high altitude forest stand, bacterial CFU ranged between 25.00-62.00 at 0-10 cm depth at. At 10-20 cm depth, it ranged between 26.00-60.00, whereas, at 0-10 cm depth at the low altitude forest stand, it ranged between 20.00-52.00. At 10-20 cm depth it ranged between 25.00-50.00.
Bacterial Species Composition and Diversity
Table 1 depicts the list of bacterial species isolated at the two different soil depths at the two forest stands during the study periods of 2009 and 2010. A total of 14 bacterial species were isolated from two different soil depths at the two forest stands. At the high altitude forest stand, 10 and 8 bacterial species were isolated from the surface and sub-surface soil layers respectively, whereas, at the low altitude forest stand, 8 and 5 bacterial species were isolated from the surface and sub-surface soil layers respectively. The common bacterial species isolated were Bacillus subtilis and Micrococcus sp. The dominant bacterial species isolated from 0-10 cm depth at the high altitude forest stand were Arthrobacter sp., Bacillus subtilis and Micrococcus luteus. At 10-20 cm depth, Azotobacter sp. and Bacillus sp. were found to be the dominant bacterial species. At the low altitude forest stand at the 0-10 cm depth, the dominant bacterial species isolated were Bacillus mycoides and B. subtilis. At the 10-20 cm depth, Azotobacter sp. and Bacillus subtilis were the dominant bacterial species. However, a few bacterial species were restricted to a particular soil depth. Acetobacter sp. and Xanthomonas sp. were restricted to 0-10 cm depth. Pseudomonas aeruginosa was restricted to 10-20 cm depth at the high altitude forest stand. Chromobacterium sp. was restricted to 0-10 cm depth and Rhizobium sp. was restricted to 10-20 cm depth at the low altitude forest stand. Shannon diversity index of bacterial species isolated at two different soil depths at the two forest stands during the study periods of 2009 and 2010 was found to be higher at 0-10 cm depth than at 10-20 cm depth. Shannon diversity index values of bacterial species isolated during the study periods ranged between 1.91-1.95 at 0-10 cm depth and 1.78-1.88 at 10-20 cm depth at the high altitude forest stand, whereas, at the low altitude forest stand, the index of diversity (Shannon) values of bacterial species isolated for both the years ranged between 1.45-1.50 at 0-10 cm depth and 1.27-1.45 at 10- 20 cm depth (Figure 2). Simpson dominance index of bacterial species at two different soils depths at the two forest stands during the study periods of 2009 and 2010 was found to be higher at 10-20 cm than at 0-10 cm depth. Simpson dominance index values for bacterial species isolated during the study periods ranged between 0.018-0.19 at 0-10 cm depth and 0.01-0.02 at 10-20 cm depth at the high altitude forest stand, whereas, at the low altitude forest stand, the index of dominance (Simpson) values of bacterial species isolated ranged between 0.01-0.02 at 0-10 cm depth and 0.02-0.03 at 10-20 cm depth at the low altitude forest stand (Figure 3). Table 2 depicts the range and mean values of the various physico-chemical properties of soil. Soil temperature, moisture content, pH, organic carbon, total nitrogen, available phosphorus and exchangeable potassium exhibited monthly variations at the two forest stands during the study periods i.e., 2009 and 2010. Except exchangeable potassium and soil temperature, all of them were higher at the high altitude forest stand than that at the low altitude forest stand. Also, except soil temperature, surface soil layer (0-10 cm depth) exhibited higher values than sub-surface soil layer (10-20 cm depth) at the two forest stands. Tables 3-6 depict the correlation coefficient (r) values of bacterial CFU and the various physico-chemical properties at 0-10 cm and 10-20 soil depths at the two different forest stands during the study period of 2009 and 2010. Table 3 depicts the correlation coefficient (r) values of bacterial CFU and the various physico-chemical properties at 0-10 cm and 10-20 cm soil depths at the high altitude forest stand during the study period of 2009. At 0-10 cm depth, bacterial CFU was positively correlated with moisture content (r=0.70; p ≤ 0.05), organic carbon (r=0.77; p ≤ 0.05) and available phosphorus (r=0.76; p ≤ 0.05), whereas, at 10-20 cm depth, bacterial CFU was positively correlated with organic carbon (r= 0.70; p ≤ 0.05) only. Table 4 depicts the correlation coefficient (r) values of bacterial CFU and the various physico-chemical properties at 0-10 cm and 10-20 cm soil depths at the low altitude forest stand during the study period of 2009. At 0-10 cm depth, bacterial CFU was positively correlated with total nitrogen (r=0.73; p ≤ 0.05) and available phosphorus (r=0.59; p ≤ 0.05), whereas, at 10-20 cm depth, bacterial CFU was positively correlated with moisture content (r=0.64; p ≤ 0.05), organic carbon (r= 0.66; p ≤ 0.05), total nitrogen (r=0.71; p ≤ 0.05) and exchangeable potassium (r=0.70; p ≤ 0.05). Table 5 depicts the correlation coefficient (r) values of bacterial CFU and the various physico-chemical properties at 0-10 cm and 10-20 cm soil depths at the high altitude forest stand during the study period of 2010. At 0-10 cm depth, bacterial CFU was positively correlated with moisture content (r=0.80; p ≤ 0.01), organic carbon (r=0.71; p ≤ 0.05), total nitrogen (r=0.58; p ≤ 0.05) and available phosphorus (r= 0.58; p ≤ 0.05). At 10-20 cm depth, bacterial CFU was positively correlated with organic carbon (r= 0.60; p ≤ 0.05) and total nitrogen (r= 0.56; p ≤ 0.05). Table 6 depicts the correlation coefficient (r) values of bacterial CFU and the various physico-chemical properties at 0-10 cm and 10-20 cm soil depths at the low altitude forest stand during the study period of 2010. At 0-10 cm depth, bacterial CFU was positively correlated with organic carbon (r=0.64; p ≤ 0.05), total nitrogen (r=0.64; p ≤ 0.05) and exchangeable potassium (r=0.65; p ≤ 0.05). At 10-20 cm depth, bacterial CFU was positively correlated with organic carbon (r=0.90; p≤ 0.001) and total nitrogen (r=0.76; p ≤ 0.05). The one-way analysis of variance (ANOVA) of various physico-chemical properties of soil showed significant variations at p ≤ 0.05 between the two soil depths at the two forest stands (Table 7).
| Sl.No. | Bacterial species | US1 | US2 | M1 | M2 |
|---|---|---|---|---|---|
| 1 | Acetobacter sp. | + | - | - | - |
| 2 | Arthrobacter sp. | + | + | + | - |
| 3 | Azotobacter sp. | + | + | - | + |
| 4 | Bacillus sp. | + | + | - | - |
| 5 | B. mycoides | - | + | + | - |
| 6 | B. subtilis | + | + | + | + |
| 7 | Chromobacterium sp. | - | - | + | - |
| 8 | Escherichia coli | + | + | + | - |
| 9 | Micrococcus sp. | + | + | + | + |
| 10 | M.luteus | + | - | + | - |
| 11 | Pseudomonas sp. | - | - | + | - |
| 12 | P.aeruginosa | - | + | - | + |
| 13 | Rhizobium sp. | + | - | - | + |
| 14 | Xanthomonas sp. | + | - | - | - |
Note: ‘+’ indicates present; ‘-’ indicates absent; US1= Upper Shillong (high altitude) 0-10 cm soil depth; US2= Upper Shillong (high altitude) 10-20 cm soil depth; M1= Mawkyrdep (low altitude) 0-10 cm soil depth; M2= Mawkyrdep (low altitude) 10-20 cm soil depth.
Table 1: List of Bacterial Species Isolated at Two Different (0-10 cm and 10-20 cm) Soil Depths at the Two Forest Stands During the Study Periods of 2009 to 2010.
| Soil properties | 2009 | 2010 | ||||||
|---|---|---|---|---|---|---|---|---|
| US1 | US2 | M1 | M2 | US1 | US2 | M1 | M2 | |
| ST (⁰C) | 8.00-18.00 (12.75 ± 0.5) | 8.00-17.50 (11.88 ± 0.2) | 12.00-24.00 (16.83 ± 0.10 | 12.00-23.00 (12.00 ± 0.03) | 7.00-17.30 (12.26 ± 0.04) | 7.00-17.00 (10.98 ± 0.1) | 13.00-24.00 (18.13 ± 0.03) | 11.00-21.90 (16.39 ± 0.02) |
| MC (%) | 23.30-49.50 (37.60± 0.54) | 21.00-48.40 (34.87 ± 0.03) | 22.00-47.00 (34.09 ± 0.03) | 18.70-43.00 (30.27 ± 0.05) | 25.00-50.47 (34.66 ± 0.02) | 21.00-49.43 (32.83 ± 0.09) | 19.58-43.83 (26.86 ± 0.08) | 17.00-40.00 (25.12 ±-0.03) |
| pH | 4.83-6.20 (5.35 ± 0.04) | 4.86-5.57 (5.30 ± 0.04) | 5.23-6.07 (5.56 ± 0.03) | 4.90-5.73 (5.40 ± 0.06) | 4.70-6.30 (5.06 ± 0.03) | 4.60-5.70 (5.09 ± 0.04) | 5.23-6.26 (5.66 ± 0.03) | 4.98-6.00 (5.40 ± 0.03) |
| OC (%) | 2.03-5.64 (3.93 ± 0.21) | 1.97-4.28 (3.39 ± 0.21) | 1.39-3.47 (3.47 ± 0.23) | 1.39-3.11 (2.08 ± 0.21) | 2.40-4.72 (3.51 ± 0.02) | 2.10-4.21 (3.42 ± 0.65) | 2.00-3.70 (3.18 ± 0.45) | 2.60-4.00 (3.28 ± 0.43) |
| TN (%) | 0.011-0.030 (0.020 ± 0.021) | 0.010-0.021 (0.014 ± 0.037) | 0.008-0.025 (0.017 ± 0.012) | 0.006-0.023 (0.015 ± 0.090) | 0.015-0.030 (0.021 ± 0.013) | 0.016-0.025 (0.018 ± 0.015) | 0.005-0.025 (0.017 ± 0.011) | 0.005-0.30 (0.014 ± 0.012) |
| AP (%) | 0.012-0.124 (0.078 ± 0.90) | 0.036-0.122 (0.075 ± 0.043) | 0.024-120 (0.080 ± 0.065) | 0.056-0.108 (0.083 ± 0.010) | 0.046-0.116 (0.073 ± 0.029) | 0.060-0.115 (0.060 ± 0.021) | 0.060-0.112 (0.089 ± 0.013) | 0.056-0.104 (0.073 ± 0.024) |
| EK (%) | 0.400-0.969 (0.691 ± 0.071) | 0.326-0.733 (0.510 ± 0.056) | 0.348-0.968 (0.682 ± 0.081) | 0.280-1.014 (0.641 ± 0.016) | 0.263-0.645 (0.465 ± 0.045) | 0.112-0.905 (0.413 ± 0.045) | 0.300-1.108 (0.787 ± 0.065) | 0.361-0.870 (0.590 ± 0.099) |
Table 2: Values (range) of Physico-chemical Properties at Two Different (0-10 cm and 10-20 cm) Soil Depths at the Two Forest Stands During the Study Periods of 2009 and 2010. Values in the Parentheses Indicate the Mean and Standard Error.
| Soil properties | ST | MC | pH | OC | TN | AP | EK | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depths(cm) | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 |
| CFU(B) | NS | NS | 0.70 | NS | NS | NS | 0.77 | 0.70 | NS | NS | 0.59 | NS | NS | NS |
| ST | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.67 | ||
| MC | NS | NS | 0.85 | 0.91 | 0.74 | NS | NS | 0.60 | NS | NS | ||||
| pH | NS | NS | NS | NS | NS | NS | NS | NS | ||||||
| OC | 0.71 | NS | NS | 0.58 | NS | NS | ||||||||
| TN | NS | 0.65 | 0.62 | NS | ||||||||||
| AP | NS | NS | ||||||||||||
Table 3: Correlation Coefficient (r) Values of Bacterial CFU and the Various Physico-chemical Properties at 0-10 cm and 10-20 Soil Depths at the High Altitude Forest Stand During the Study Period of 2009.
| Soil properties | ST | MC | pH | OC | TN | AP | EK | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depths(cm) | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 |
| CFU(B) | NS | NS | 0.64 | NS | NS | NS | 0.66 | 0.73 | 0.71 | 0.59 | NS | NS | 0.70 | |
| ST | NS | NS | NS | NS | NS | NS | NS | -0.67 | NS | NS | NS | NS | ||
| MC | NS | NS | 0.80 | 0.95 | NS | NS | NS | NS | NS | NS | ||||
| pH | NS | NS | NS | NS | NS | NS | NS | NS | ||||||
| OC | NS | NS | NS | NS | NS | NS | ||||||||
| TN | 0.66 | NS | NS | NS | ||||||||||
| AP | NS | NS | ||||||||||||
Table 4: Correlation Coefficient (r) Values of Bacterial CFU and the Various Physico-chemical Properties at 0-10 cm and 10-20 Soil Depths at the Low Altitude Forest Stand During the Study Period of 2009.
| Soil properties | ST | MC | pH | OC | TN | AP | EK | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depths(cm) | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 |
| CFU(B) | NS | NS | 0.80 | NS | NS | NS | 0.71 | 0.60 | 0.58 | 0.56 | 0.58 | NS | NS | NS |
| ST | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
| MC | NS | NS | -0.67 | NS | NS | NS | NS | NS | NS | NS | NS | |||
| pH | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||||
| OC | NS | NS | NS | NS | NS | NS | ||||||||
| TN | NS | NS | NS | NS | ||||||||||
| AP | NS | NS | ||||||||||||
Table 5: Correlation Coefficient (r) Values of Bacterial CFU and the Various Physico-chemical Properties at 0-10 and 10-20 cm Soil Depths at the High Altitude Forest Stand During the Study Period of 2010.
| Soil properties | ST | MC | pH | OC | TN | AP | EK | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depths(cm) | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 |
| CFU(B) | NS | NS | NS | NS | NS | NS | 0.64 | 0.90 | 0.64 | 0.76 | NS | NS | 0.65 | NS |
| ST | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
| MC | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||
| pH | NS | NS | NS | NS | NS | NS | NS | NS | ||||||
| OC | 0.58 | 0.68 | NS | NS | NS | NS | ||||||||
| TN | NS | NS | NS | NS | ||||||||||
| AP | NS | NS | ||||||||||||
Table 6: Correlation Coefficient (r) Values of Bacterial CFU and the Various Physico-chemical Properties at 0-10 cm and 10-20 Soil Depths at the Low Altitude Forest Stand During the Study Period of 2010.
| Physico-chemical properties of soil | Sources of variation | 2009 | 2010 | ||
|---|---|---|---|---|---|
| F-value | P-level | F-value | P-level | ||
| ST (⁰C) | US1XUS2XM1XM2 | 946.5521 | 0.000000 | 873.8466 | 0.000000 |
| US1 X M1 | 470.0256 | 0.000000 | 475.9691 | 0.000000 | |
| US2 X M2 | - | - | 398.9938 | 0.000000 | |
| MC (%) | US1XUS2XM1XM2 | 827.5211 | 0.000000 | 544.7468 | 0.000000 |
| US1 X M1 | - | - | 285.1836 | 0.000000 | |
| US2 X M2 | 428.1493 | 0.000000 | 260.7996 | 0.000000 | |
| pH | US1XUS2XM1XM2 | - | - | 12176.31 | 0.000000 |
| US1 X M1 | 8891.396 | 0.000000 | 5197.311 | 0.000000 | |
| US2 X M2 | - | - | 7377.244 | 0.000000 | |
| OC (%) | US1XUS2XM1XM2 | 606.8623 | 0.000000 | - | - |
| US1 X M1 | 281.5642 | 0.000000 | - | - | |
| US2 X M2 | 341.9304 | 0.000000 | - | - | |
| TN (%) | US1XUS2XM1XM2 | 578.6198 | 0.000000 | 465.8336 | 0.000000 |
| US1 X M1 | - | - | - | - | |
| US2 X M2 | - | - | - | - | |
| AP (%) | US1XUS2XM1XM2 | - | - | 598.0976 | 0.000000 |
| US1 X M1 | - | - | - | - | |
| US2 X M2 | - | - | 323.1483 | 0.000000 | |
| EK (%) | US1XUS2XM1XM2 | 212.9581 | 0.000000 | 513.2905 | 0.000000 |
| US1 X M1 | 11.3050 | 0.002813 | 248.5344 | 0.000000 | |
| US2 X M2 | - | - | 308.4816 | 0.000000 | |
Note: US1: Upper Shillong (high altitude) at 0-10 cm soil depth; US2: Upper Shillong (high altitude) at 10-20 cm soil depth; M1: Mawkyrdep (low altitude) at 0-10 cm soil depth; M2: Mawkyrdep (low altitude) at 10-20 cm soil depth; ST: Soil Temperature; MC: Moisture Content; OC: Organic carbon; TN: Total Nitrogen; AP: Available Phosphorus; EK: Exchangeable Potassium. Insignificant values are marked with &lsquo-’.
Table 7: One Way Analysis of Variance (ANOVA at p ≤ 0.05) of Physico-chemical Properties at Two Different (0-10 cm and 10-20 cm) Soil Depths at the Two Forest Stands During the Study Periods of 2009 and 2010.
Soil temperature
Variation in the soil temperature of high and low altitudes is in conformation with the altitude of the two forest stands respectively, where the temperature of the soil is affected by the altitude of the land, slope and also by the climate of the particular place. It may also be due to the increase in absorption of solar radiation by mineral soil due to removal of forest cover observed at the low altitude forest stand, a state of deforestation which has led to the warming of the soil which in turn causes increase of the soil temperatures. This is in consistent with the earlier report of Hashimoto and Suzuki [11]. Increase soil temperature at the two study sites during the summer months could be due to effect of the solar radiation and the heating up of the surrounding soil surface.
Moisture content
The higher soil moisture content at the high altitude forest stand can be attributed to a combination of the higher infiltration rate allowing more water into the profile in the high altitude soil, as well as the water extraction by the plants. It could also be due to the high organic matter content, dense herbaceous vegetation and low atmospheric temperature which may have helped retaining more moisture in the soil. This is in conformity with the findings of Elliott et al. [12]; Tejedor et al. [13]. The lower moisture content at the low altitude forest soil was the result of quick run-off from the slopes and low water retention capacity of the soil.
pH
The high acidic nature of soil at the high altitude forest stand may be due to the thickness of the forest and also accumulation of leaf litters on the forest floor.
Organic carbon
Soil at the high altitude forest stand showed higher organic carbon than at the low altitude forest stand. This may be due to the dense vegetation which resulted in accumulation of litters. Higher soil organic carbon at the surface layer is due to the fact that organic residues are usually incorporated in the surface soil and the leftover residues of shallow-rooted plants also get accumulated in the top few centimeters of the soil Sudhir and Siddaramppa [14]. Deforestation is one of the prominent anthropogenic disturbances at the low altitude forest stand. This resulted in opening up of forest canopy and alterations in the forest-floor microenvironment that deteriorates the soil nutrient level. The higher concentration of nutrients in surface (0-10 cm) soil layer might be due to higher organic matter content in the surface layer. Surface layer is continuously enriched by the nutrients released from decomposing litters. In addition to regulating the oxygen content of the soil, moisture partly regulates the availability and movement of nutrients to the microbes. Greater organic carbon content in soils at high altitude forest indicated more microbial biomass, which may be due to increased moisture content. The finding of the present study was in conformity with the work of Arunachalam and Pandey, Mishra and Laloo [15]; Mishra [16]. Amount and quality of plant residues can also influence the composition of the soil microbial community as well as the dynamics of carbon and nutrient release in soil Nelson and Mele [17]. Fine particles (clay) help in retention of more organic C, N and K in soil of high altitude forest stand Mishra [18].
Total nitrogen
The higher nitrogen content of soil at the high altitude forest stand is also probably due to the organic matter and leaf litter accumulation in the forest floor. Higher content of nitrogen at the surface soil layer could be ascribed to the higher amount of organic matter at the surface soil layer as it leads to the increment of mineralisable nitrogen and phosphorus. Reiners et al. [19] also reported that the higher levels of carbon and nitrogen, the two important nutrients for plant growth and function occur in the surface soil layer. Similar results with positive correlation of organic carbon to nitrogen and phosphorus have been reported by other researchers Rezende et al. [20]; Verma and Sweta [21].
Available phosphorus
Higher concentration of available phosphorus at the high altitude forest stand could be related to higher microbial activity. Tiwari et al. [22] suggested that the monthly variations may be related to the rapid release of this nutrient from the litter at the same period. There was not much difference in available phosphorus of soil at both the soil depths; however, slight decrease in available phosphorus with increase in soil depth was observed which can be due to the organic matter distribution which is usually higher at the surface soil layer. Phosphate is relatively immobile and this may be the reason for the little variations noted along the depths. Phosphorus generally accumulated to the surface soil layer. Similar findings were also reported by Munro et al. [23]; Gadermaier et al. [24].
Exchangeable potassium
In the present study, exchangeable potassium was higher at the low altitude forest stand. This could be due to the topography of the forest stand. Almost all high land soils suffered from potassium losses due to leaching and soil erosion. Potassium content of the soil also depends on the topography as well as on the quality of the soil. It decreases with increase in soil depth in most of the months during the study periods which might be due to the absorption of nutrients by the plants. Gadermaier et al., [24] also reported similar findings that the soil potassium was higher in the surface soil suggesting that it gets accumulated at the surface soil layer and it is closely related to the organic carbon of the soil.
Bacterial population was positively correlated with organic carbon and total nitrogen and it can be concluded that these two constitute the major driving factors of bacterial population in both the two forest stands of Meghalaya.
The authors thank the Head, Department of Botany, North-Eastern Hill University, Shillong, India for providing the necessary laboratory facilities. The first author is deeply grateful to DBT and CAS, North-Eastern Hill University for providing necessary instruments and the University Grants Commission-Rajiv Gandhi National Fellowship for SC/ST, New Delhi for giving financial support to undertake this investigation.