Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2019)Volume 7, Issue 2
Background: Atherogenic progression in Coronary Artery Disease (CAD) involves a chronic inflammatory process, where local production or release of pro-inflammatory mediators into coronary circulation has a main role. Proinflammatory cytokines IL-6, TNFα and IL1β have been particularly related with the atheroma plaque and CAD severity.
Aim: To explore whether characteristics of the coronary artery affected by atheroma plaque were related with coronary circulating pro-inflammatory cytokines and their modifications after angioplasty.
Methods: Patients with CAD and stable coronary plaque, submitted to coronary angiography and stenting. Coronary artery lumen diameter at the site of the main atherogenic lesion was measured by intravascular ultrasound, whereas IL-6, TNFα and IL1β concentration in coronary and peripheral blood samples, either before or after angioplasty were determined by multiplexing assay. Baseline distribution of coronary cytokines, or its modification after angioplasty, was evaluated according to the diameter of the lumen of coronary artery (subgroups divided by cutoff value 3.5 mm).
Results: Coronary lumen diameter <3.5 mm was related to higher baseline coronary concentrations of IL-1β; as well as to the significant increase in coronary concentrations of TNF-α and IL-6, after angioplasty. Baseline cytokines at peripheral blood did were not modified by coronary lumen diameter; however, coronary lumen <3.5 mm was associated to a peripheral increase in IL-1β, after angioplasty.
Conclusion: Coronary lumen diameter, possibly implying plaque characteristics, affects baseline and post-angioplasty coronary concentrations of pro-inflammatory cytokines in patients with CAD.
Coronary Artery Diseases; Atherogenesis; Inflammation; Cytokines; Intravascular ultrasound; Lumen Diameter; Angioplasty
Coronary Artery Disease (CAD) involves a chronic inflammatory process with the participation of multiple cell types in various stages of activation, apoptosis and necrosis. Monocytes/macrophages, lymphocytes, endothelial cells and vascular smooth muscle cells are activated by a complex network of pro-inflammatory cytokines induced by an hyperlipidemic environment, accompanied by a maladaptive response, resulting in a localized vascular inflammation that eventually translates in negative remodeling and progression of the atherosclerotic plaque [1].
Several mechanisms participate during CAD pro-atherogenic process, involving lipoprotein retention, inflammatory cell recruitment, foam cell formation, apoptosis and necrosis, smooth muscle cell proliferation, extracellular matrix synthesis, calcification, angiogenesis, arterial remodeling, fibrous cap rupture and thrombosis [2].
Atherosclerosis produces structural changes in the artery wall; which primarily affects the intimate layer, producing accumulation of cholesterol, cell elements and their metabolic products. On the other hand, vascular smooth muscle cells in the artery middle layer differentiate and migrate to the intima, disturbing the vascular tone regulation at the injured area [3].
As coronary atherogenesis progresses, the atheroma plaque slowly grows within the coronary artery wall, along the local accumulation of a large amount of thrombogenic content, which eventually reduces the coronary lumen and limits myocardial oxygen supply [4]. Atherogenic progression depends on genetics and metabolic components, as well as local coronary factors, such as the release of pro-inflammatory and pro-atherogenic mediators [5,6]. In this context, pro-inflammatory cytokines IL-6, TNFα and IL1β have been particularly related with the atheroma plaque and CAD severity [7-9]. Therefore, the present study aimed to explore whether characteristics of the coronary artery affected by atheroma plaque were related with coronary circulating pro-inflammatory cytokines and their modifications after angioplasty.
Design
This is an investigator-driven, quasi-experimental, prospective, observational study. Primary endpoint was the difference of coronary circulating pro-inflammatory cytokines, or its modification after angioplasty, according to the characteristics of the coronary artery affected by atheroma plaque.
Study population
Patients older than 18 years with post-infarction angina, candidates for coronary angiography and stenting, with chronic CAD and stable coronary plaque, obstructing ≥ 50% of the coronary lumen, admitted to the Hemodynamics Department from the Centro Médico Nacional “20 de Noviembre” ISSSTE, a tertiary referral hospital in Mexico City. This study was conducted in collaboration with the Experimental Metabolism and Clinical Research Laboratory, Centro Médico Nacional “20 de Noviembre” ISSSTE. The study complied with the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments, and was approved by the local Institutional Committees of Research, Ethics and Biosafety (Protocol ID No. 096.2014). All participants provided written informed consent.
Angiography, intravascular ultrasound, stenting and sample collection
Coronary angiography and stenting were carried out according to standard techniques using radial approach. Evaluable vessels were larger than 1.5 mm and ≥ 50% obstruction of the coronary lumen. The affected area of the coronary artery was assessed with intravascular ultrasound, carried out after the administration of heparin to avoid coronary spasms. The measure of the coronary artery lumen diameter corresponds to the smallest luminal area found at the site of the main atherogenic lesion, observed transversally. Measures were performed using VOLCANO software. And a cut-off median value of the arterial lumen (3.5 mm) was determined for the degree of the injury. Coronary stenting was performed using drug-eluting stents (drug eluted was everolimus in 50% of the cases and zotarolimus in the remaining cases). The mean number of stents applied was 1 per vessel, while the size and length of the stent was determined by the cardiology expert, being 3 mm diameter, and 20 mm length stents the most frequently used. Standard dual antiplatelet therapy previous to Percutaneous Coronary Intervention (PCI) consisted of aspirin 150 mg/day and clopidogrel 75 mg/day in patients with chronic stable angina. For blood sampling, 6 mL of coronary blood was collected before angioplasty from the closest location to the atheroma plaque; and after the angioplasty blood was from coronary sinus. Brachial peripheral blood samples were also acquired at the same times for comparative analysis.
Immuno-magnetic assay
Pro-inflammatory plasma biomarkers IL-1β, IL-6, TNF-α were determined by immuno-magnetic multiplexing assay, (MILLIPLEX MAP Human TH17 Magnetic Bead Panel) and read in MAGPIX System 40-072-EM Millipore, Austin, Texas, following recommendations of the provider.
Statistical analysis
All continuous variables were expressed as the mean ± Standard Deviation (SD). Data distributions for the variables were estimated using K-S test. Independent (1-tailed) Mann-Whithney test were applied for comparison between mean values. All statistical analyses were performed using SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA) for Windows®, and p values ≤ 0.05 were considered to be statistically significant.
Ten patients with CAD and stable coronary plaque, mean aged 68 years old (7 males, 3 females) with most prevalent comorbidities of high blood pressure (70%), dyslipidemia (70%) and type 2 Diabetes Mellitus (50%) were included. During coronary angiography, intravascular ultrasound was used to determine coronary artery lumen diameter at the site of the main atherogenic lesion, observing obstructions in a range of 50% to 75%, and coronary lumen diameter ranging 2.7 to 4.8 mm. Then, pro-inflammatory cytokines circulating in coronary blood, either before or after angioplasty, were determined. To evaluate cytokines relation with characteristics of the coronary artery affected by atheroma plaque, the study population was divided according to a cut-off median value of 3.5 mm of coronary lumen diameter, and distribution of pro-inflammatory cytokines, or its modification after angioplasty, were compared between subgroups. Representative intravascular images are shown in Figure 1.
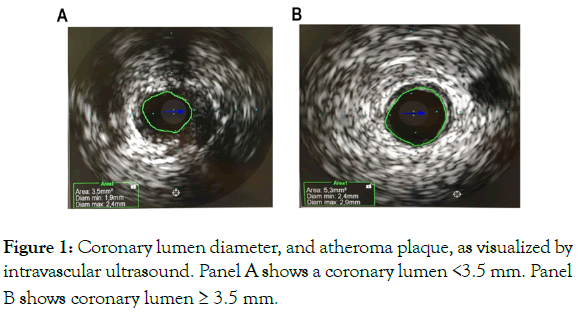
Figure 1: Coronary lumen diameter, and atheroma plaque, as visualized by intravascular ultrasound. Panel A shows a coronary lumen <3.5 mm. Panel B shows coronary lumen ≥ 3.5 mm.
Coronary lumen diameter <3.5 mm was related to higher baseline coronary concentrations of IL-1β (Figure 2); as well as the significant increase in coronary concentrations of TNF-α and IL-6, after angioplasty (Figure 3).
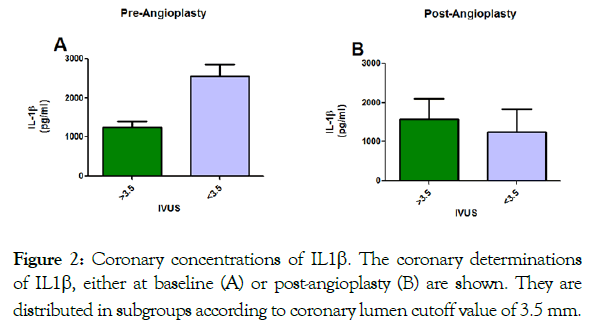
Figure 2: Coronary concentrations of IL1β. The coronary determinations of IL1β, either at baseline (A) or post-angioplasty (B) are shown. They are distributed in subgroups according to coronary lumen cutoff value of 3.5 mm.
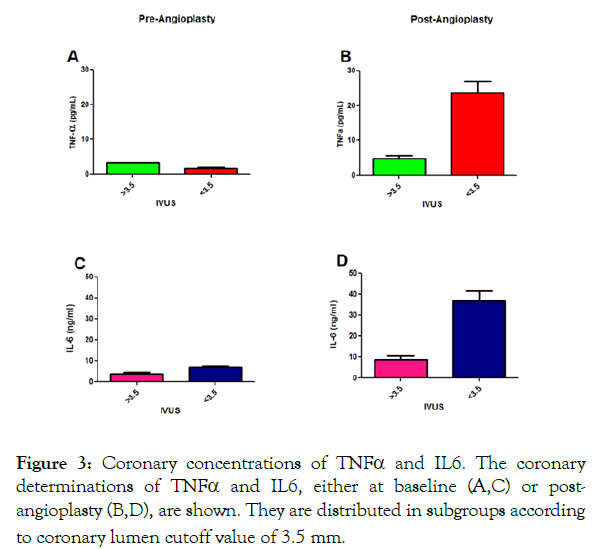
Figure 3: Coronary concentrations of TNFα and IL6. The coronary determinations of TNFα and IL6, either at baseline (A,C) or postangioplasty (B,D), are shown. They are distributed in subgroups according to coronary lumen cutoff value of 3.5 mm.
Baseline cytokines at peripheral blood did were not modified by coronary lumen diameter; however, coronary lumen <3.5 mm was associated to a peripheral increase in IL-1β, after angioplasty.
The main finding of the present study was that the diameter of coronary lumen influences baseline and post-angioplasty coronary concentrations or pro-inflammatory cytokines.
Previous studies had shown higher blood concentrations of TNF-α associated with more severe coronary lesions, assessed by intravascular ultrasound, in patients with stable angina [10]; however, our study provides a more complete mechanistic description, including coronary concentrations of pro-inflammatory cytokines and angioplasty-related modifications.
The diameter of coronary lumen may be associated with the plaque size and/or composition, which affect coronary lumen. We found that lower coronary lumen, potentially implying higher atheroma plaque, was related with higher coronary concentration of IL-1β. Consistently, increased IL-1β has been involved in the development of the plaque and clinical complications [11]. On the other hand, IL-1β is expressed in the foam cells of the intima [11], which may explain the lack of further increase after angioplasty.
Nevertheless, post-angioplasty increase of TNF-α and IL-6 in cases of lower coronary lumen suggests that pro-inflammatory mediators like TNF-α and IL-6 are associated to the plaque composition; and when the plaque is impacted by balloon angioplasty, cytokines sequestered within the plaque would be released into coronary blood flow. Consistently, addition of Eicosapentaenoic Acid, which exerts effects on plaque components, to strong statin therapy, results in modifications of pro-inflammatory cytokines [12].
Finally, coronary lumen diameter does not seem to influence peripheral cytokines, whereas increase in peripheral IL1b after angioplasty may occur due to a different mechanism. Some limitations of the present study include: 1) The low number of patients, and 2) The limited number of samples obtained during the time course after angioplasty. Therefore, further and larger prospective studies exploring coronary plaque characteristics affecting the release of pro-inflammatory cytokines, and their modifications after angioplasty and stenting, in are guaranteed.
Coronary lumen diameter, possibly implying plaque characteristics, affects baseline and post-angioplasty coronary concentrations of pro-inflammatory cytokines in patients with CAD.
The authors thank the support of Institutional Program E015; and Fondo Sectorial FOSSIS-CONACYT, SALUD-2014-1-233947.
The author’s thank Miss Cristina Rubio for her expert administrative and logistic assistant, Ernesto Fernández-Ceseña, and Hector Hugo Escutia-Cuevas for the facilitation of blood sample, and Guillermo Garcia-Castillo for the facilitation in the flow cytometer use.
Citation: Suarez-Cuenca JA, Vera-Gomez E, Hernandez-Patricio A, Gutierrez-Buendia JA, Dominguez-Perez GA GA, Robledo-Nolasco R, et al. (2019) Relation of Coronary Artery Lumen with Baseline, Post-angioplasty Coronary Circulating Pro-Inflammatory Cytokines in Patients with Coronary Artery Disease. Angiol Open Access 7:226.
Received: 11-May-2019 Accepted: 21-May-2019 Published: 25-May-2019 , DOI: 10.35248/2329-9495.19.7.227
Copyright: © 2019 Suarez-Cuenca JA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.