Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2023)Volume 14, Issue 6
Background: Hybrid Cooperative Complex (HCC) is a novel molecule, launched worldwide in 2016, which contains ultrapure high and low molecular weight hyaluronic acid stabilized in a thermal process and without chemical additives, and it is indicated for tissue remodeling and improvement of sagging skin. It is proved that HCC can stimulate the production of elastin and collagen by keratinocytes and fibroblasts, differentiation of steam cells into adipocytes, and accelerate the wound healing process. It is currently described for treating the mid and lower third of the face, but not the upper third. Its great integration capacity and uniform diffusion are favorable characteristics for the forehead and periorbital region, with tissues frequently adhered to the underlying musculature.
Objective: To evaluate the effectiveness and safety of HCC in the upper third of the face and to describe a novel technique of application.
Methods: Nine subjects received HCC treatment in the forehead and periorbital regions, two applications with a 4- week interval, with a 22 gauge microcannula technique, under anesthesia. Photographs with Vectra® Software were taken and a non-involved doctor compared the photographs taken and used de Global Aesthetic Improvement Scale (GAIS) score to classify. Subjects were asked in the end of study about perception of improveness in luminosity and hydration of forehead and periorbital skin. This is the first study involving the treatment of the upper third of the face with HCC.
Results: The GAIS score was 2 after the 9th week; 56% of the subjects noticed improvement of hydration, and 78% noticed improvement of luminosity. No important adverse events happened. Seven out of nine of the patients would recommend the treatment and would repeat it.
Conclusion: HCC is a safe and effective treatment option for the upper third of the face. Further studies should be conducted to understand the long-term performance of the product.
Hyaluronic acid; Cellular senescence; Skin aging; Forehead
Facial rejuvenation of the upper third of the face has improved with non-surgical techniques in the past two decades, like neurotoxins, facial fillers, and energy-based devices [1]. The approach to this topography should involve profound knowledge of anatomy, physiology, and different treatment options for each facial structure [2].
Depressors and elevators muscles of the face are involved in facial mimicry. Separated in the mid-facial line, the frontalis muscles are the only elevators, which contraction happens mostly in their lower third, leading to horizontal rhytids on the forehead. Each one is covered by aponeurotic galea and inserts in the orbicularis oculi muscles. The aponeurotic galea continues to the lateral portion of the superficial temporal fascia, where the periosteum of the frontal bone is continuous to the deep temporal fascia. These fascial layers converge at a spot medial to the cranial temporal crest. There are four depressor muscles in the upper third of the face: Procerus, corrugators, eyebrow depressors and the orbital portion of the orbicularis orris, which when contracting causes the glabellar rhytids.
Most of the region’s innervation is supplied by the supraorbital and supratrochlear nerves. The superficial branches pass through the surface of the frontalis, being responsible for the touch on the forehead and anterior scalp. Their lesion could cause eyebrow ptosis and asymmetries on the frontalis. In the upper third of the face, their branches run close to the superficial temporal fascia. So, the safest planes to treat the forehead considering protection to the temporal branch of the facial nerve are the supraperiosteal and subdermal [2].
The vascular anatomy of the forehead is extremely variable, with frequent asymmetries in the same individual. The internal carotid artery originates the ophthalmic artery, posterior to the eyeball, which gives rise to the supratrochlear, supraorbital, dorsal nasal and lacrimal arteries. The supratrochlear artery superficializes 15 to 25 millimeters above the orbital rim. The supraorbital artery emerges in the same-named foramen and goes to the forehead in an oblique path, anastomosing with the superficial temporal artery in the lateral orbital rim. Similarly to the supratrochlear, the supraorbital also superficializes 15 mm to 25 mm above the orbital rim. The supraorbital and supratrochlear arteries are the most related to occlusive complications due to facial fillers [3]. Skin aging clinically manifests with thinning, dryness, rhytids and loss of elasticity.
Histologically, it is characterized by an accumulation of elastotic material in the papillary and middle dermis, a reduction in the concentration of hyaluronic acid and a decrease in collagen biosynthesis [1,4,5]. Extrinsic and intrinsic factors contribute to the appearance of such alterations. Non-surgical procedures, such as chemical peelings and lasers, promote epidermal regeneration and stimulate the production of collagen and proteoglycans. Collagen biostimulators [6,7] skin boosters, hyaluronic acid fillers, energy-based devices such as microfocused ultrasound, radiofrequency and lasers [3] contribute to neocollagenesis and elastic fiber formation. They have been described in the literature as options for facial rejuvenation. When it comes to myomodulation of the mimic muscles, botulinum toxin is configured as the gold standard treatment through temporary muscle relaxation. It is the most used treatment for the upper third, often in monotherapy [8,9].
Hyaluronic Acid (HA) helps to hydrate the skin and create a stable extracellular matrix, which is a supportive structure that is essential for the normal function of the keratinocytes in epidermis and fibroblasts in the dermis. The injection of HA cross-linked or non-cross-linked in dermis can be used to improve skin quality. While non-reticulated HA is known to its low durability because of rapid degradation by endogenous enzymes, reticulated HA is related to greater ranges of adverse effects, like lumpiness, nodules, vessel occlusion and extrusion of the filler material [10].
Profhilo® is a product based on stable, Hybrid and Cooperative Complexes (HCC) produced by means of NAHYCO® Hybrid Technology, which is an innovative thermal process that rules out the use of any chemical reagents. It results in filler with low viscosity and high biocompatibility that favors optimal diffusion at the tissue level to obtain the target bio remodeling of the skin. It differs from others also because of its high concentration of hyaluronic acid (32 mg/mL) and no association with inflammatory response [11].
It is indicated for tissue remodeling and improvement of sagging skin on the face, neck and body. Its rheological profile allows tissue remodeling with 2 applications with an interval of 4 weeks. The Bio Aesthetic Points technique (BAP), for the middle and lower third of the face, is the most recommended by the manufacturer and approved for use in Brazil.
Profhilo® can improve skin hydration by 29%, compliance by 25.1% and elasticity by 47.5%, in addition to activating the adipogenic differentiation of mesenchymal stem cells and stimulating up to 12 times more production of collagen I, III, V, VII and elastin compared to other high and low molecular weight cross-linked hyaluronic acids [12].
CHC has optimistic characteristics for use in the upper third of the face due to its integration capacity and uniform diffusion, favorable characteristics for the forehead region, with tissues frequently adhered to the underlying musculature [13].
There is a single report by the manufacturer of a treatment of a 64-year-old patient with a combination of botulinum toxin and Profhilo®, with satisfactory results reported by the patient after 6 months through a questionnaire. However, there is a lack of scientific studies to assess the efficacy and safety of Profhilo® as a skin bioremodeling agent in the upper third of the face [11].
This is a prospective study. All procedures were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration.
The study was approved by the Ethics Committee, protocol number 5.606.112. Ten subjects voluntarily participated in a clinical trial, wherein they signed terms of agreement and authorized the use of their image.
Four experts in minimally invasive injectable procedures were assembled in order to describe a recommendation on upper face treatment with Profhilo®.
We report nine cases of upper face rejuvenation with Cooperative Hybrid Complexes (CHC). Subjects were selected, aged 30-57 years old. Nine were female and one was male, with visible signs of aging skin in the forehead and periocular areas, like skin dryness, loss of elasticity and fine lines. The study was carried out at Instituto Boggio, located in Sao Paulo, Brazil, between October and December 2022.
The exclusion criteria included previous facial surgery, injectable procedures or energy based devices in the last 6 months, autoimmune diseases, hyaluronidase allergy background, pregnancy or breastfeeding, active skin diseases on application sites, or unreal expectations about results.
Photographic records using Vectra® Software (Canfield NJ, USA) and a questionnaire of perception of skin hydration and luminosity were applied to the subjects at three time points: Before the intervention (S0), two weeks after all interventions (S7), four weeks after all interventions (S9).
The enrolled subjects ranged in age from 27 to 57 years (median age, 36 years) and had no relevant medical history. Eight were female, and one was male, with a BMI ranging between 19-23.
Patient assessment
One patient discontinued the study due to personal issues. The subjects underwent two identical interventions, with a four-week interval between each one. Each intervention involved the administration of 0.5 mL of CHC on each side of the forehead and 0.5 mL of CHC on each side of the periorbits, totaling 2 mL per intervention, which corresponds to one syringe. In literature, there are 12 adverse spontaneous event reports and none were considered serious. Early-onset injection site reactions, i.e., swelling, edema, redness, ecchymosis, and erythema, were the most frequent. Late-onset local reactions (e.g., swelling, nodules) followed.
The technique involved a 22 Gauge microcannula, 50 mm length, for the fanning technique. There were two entry points per side for the microcannulas: One in the frontotemporal crest topography, enabling assessment of the frontal region, and the second one in the inferolateral orbital topography, enabling assessment of the lateral periorbital region, as described by Faria, et al [14].After proper facial sanitizing, all the patients received anesthesia with a 2% lidocaine solution at the entry points, in a volume of 0.5 mL per point. The microcannula was introduced in a justadermal plane and each linear retroinjection was performed using 0.1 mL of Profhilo® along each of the 5 previously plotted vectors over the periorbital and frontalis areas, totaling a volume of 2 mL per patient.
Once the access points were defined, five vectors extending 5 cm each were drawn with a plotting pencil in a way that the entire lateral periorbital and frontal regions to be treated were filled in. The angles between vectors were 15º (Figure 1). The photographs were taken with Vectra®, following recommended parameters and standards.

Figure 1: The entry points are demonstrated with a red circle: One is located in the front temporal crest topography, enabling assessment of the frontal region, and the second one in the inferolateral orbital topography, enabling the assessment of the lateral periorbital region. Five vectors extending 5 cm each were drawn with a white plotting pencil in a way that the entire lateral periorbital and frontal regions to be treated were filled in. The angles between vectors are 15°.
A non-involved doctor compared the photographs taken and used de Global Aesthetic Improvement Scale (GAIS) Score, using the scale: 1=Exceptional improvement; 2=Very improved patient; 3=Improved patient; 4=Unaltered patient; 5=Worsened patient (Table 1).
| Patient | Sex | Age | BMI | Tabagism | GAIS Scale | Would repeat? | Indicate to other? |
|---|---|---|---|---|---|---|---|
| 1 | female | 27 | 19 | no | 2 | no | no |
| 2 | female | 28 | 21 | no | 2 | yes | yes |
| 3 | female | 29 | 22 | no | 2 | yes | yes |
| 4 | female | 30 | 23 | no | 1 | yes | yes |
| 5 | female | 36 | 20,5 | yes | 2 | yes | yes |
| 6 | female | 51 | 22,2 | no | 2 | yes | yes |
| 7 | female | 53 | 20,3 | yes | 2 | yes | yes |
| 8 | female | 57 | 22,9 | yes | 2 | yes | yes |
| 9 | male | 51 | 22,8 | no | 3 | yes | yes |
Table 1: Epidemiologic characteristics of the patients, their respective GAIS classification by an external doctor a month after interventions, and satisfaction with the procedure.
At the end of the study, patients answered if they noticed improvements in skin hydration and luminosity, and whether they would consider repeating the treatment or recommending it to others.
Patients reported mild pain during treatment, but no immediate or subsequent adverse effects were observed. Edema and minor bruises were reported, which resolved quickly without requiring further interventions. While Profhilo® is typically applied with a 29G needle, it was discussed that using a microcannula instead of a needle would be safer due to the complex anatomy of the facial upper third, which contains multiple dangerous vascular zones that can lead to complications such as blindness if hyaluronans are injected intravascularly.
Due to the rheology of the product, which has a high diffusion capacity through tissues and its mechanism of action, which involves interaction with CD44 receptor in epidermal keratinocytes, dermal fibroblasts and interaction with the mesenchymal stem cells of the subcutaneous fat pads, it is important that the application plane is justadermal. Applying it in a submuscular plane, which is commonly used for other injectable treatments, would hinder integration with the target tissues. Conversely, the subdermal plane contains important arteries such as the supraorbital and supratrochlear arteries, typically located approximately 2 cm above their respective foramina. Therefore, the safest and most effective application technique for forehead assessment involves the justadermal plane reached with a microcannula. The entry point chosen for reference is the frontotemporal crest, allowing the microcannula to reach the region completely perpendicular to the supraorbital and supratrochlear arteries, minimizing the risk of artery cannulation.
The periorbital region was accessed safely using an inferolateral orbital entry point in the justadermal plane. This entry point has the additional advantage of accumulating a significant amount of the product in the inferolateral eyelid, as it is the convergence point of the retroinjections, leading to improved eyelid laxity.
It is not recommended to inject anesthesia solutions, like lidocaine with sodium bicarbonate, in regions other than the entry point, as the volume would reduce contact between the product and the dermis and epidermis, and the sodium bicarbonate could interfere with regional pH and cellular
In one of the patients, randomly selected, cutaneous ultrasound with Doppler was performed to visualize the assessment plane and the behavior of the product. It confirmed that the justadermal plane injection was achieved in both the periorbital and forehead regions, and the product exhibited excellent spreadability after 5 minutes of injection, further demonstrating its suitability for the treated regions and reducing the risk of complications such as nodules (Figure 2).
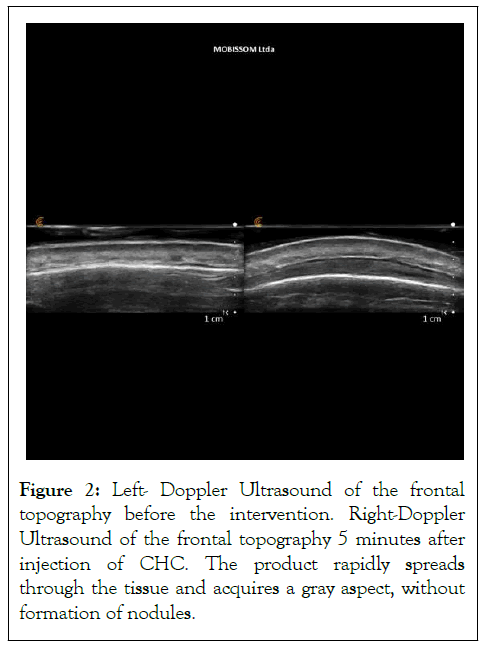
Figure 2: Left- Doppler Ultrasound of the frontal topography before the intervention. Right-Doppler Ultrasound of the frontal topography 5 minutes after injection of CHC. The product rapidly spreads through the tissue and acquires a gray aspect, without formation of nodules.
In the photographs, it was noted a reduction in the formation of wrinkles on the forehead and periorbital areas, which improved after every application. Some particularities were observed: The male patient (Figure 3), who had deep inferolateral orbital wrinkles, showed visible improvement a month after the treatment (Figures 3a-3c). A patient with acne scars showed important improvement of a deep scar on the topography of the right eyebrow after the interventions (Figure 3d). Frontal skin quality was also improved and it is most easily seen in muscle contraction (Figure 3e). In all patients, the improvement of the inferior eyelids is visible.
Figure 3: From left to right: Before interventions (W0), 2 weeks after first intervention (W2), 5 weeks after second intervention (W9). a: Male, 51 years old, with important improvement of skin quality of frontalis and eyelid. b and c: Male, 51 years old with important improvement of inferolateral wrinkles. d: Female, 28 years old, with important improvement of global skin quality, and a deep acne scar (red arrow). e: Female, 31 years old, with great improvement of wrinkle appearance and skin quality in contraction.
The average Global Aesthetic Improvement Scale score (GAIS) was 2 at the 9th week, one month after the last intervention, when photographs were evaluated by a non-involved doctor. Five out of nine subjects (56%) noticed an improvement in hydration, and seven out of nine subjects (78%) noticed an improvement in luminosity one month after the two interventions. Eight out of nine patients would recommend the treatment to a friend and would consider repeating it (Figure 4).
Figure 4: Perception of hydration improvement. Five out of nine subjects (56%) noticed an improvement in hydration; Perception
of luminosity improvement. Seven out of nine subjects (78%) noticed an improvement in luminosity one month after the two
interventions; Patient’s satisfaction: Eight out of nine patients would recommend the treatment to a friend and would consider
repeating it. Note:  : Yes;
: Yes;  : No.
: No.
HCC® has demonstrated to be a safe and effective treatment option for the upper third of the face. In the periorbital and frontalis regions, where the subcutaneous layer is thinner compared to the middle and lower facial thirds and considering the vascular characteristics and adherence of tissues to the frontal muscles, the use of a micro cannula at the specified entry points proves to be a safe and effective method.
The cutaneous ultrasound was executed By Md Luciana Zattar.
• The authors declare that they have no conflicts of interest to disclose for this paper. Juliana Palo is speaker for Profhilo. Gladstone Faria is speaker for Merz. Ricardo Boggio is speaker for Allergan.
• The study was duly submitted to the Research Ethics Committee under number 61450122.4.0000.9487 and favorable opinion to conduct the study under number 5.606.112.
• All participants signed the free and informed consent form.
Pol-Lux® Brazil funded the Profhilo® syringes and the Cutometer and Corneometer. Vectra® was landed by Medsystems®. Despite this support, this study was conducted with autonomy and independence by the authors, and Pol- Lux® and Medsystems® had any participation or influence to design, conduct, collect, assess and evaluate the presented data.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Palo JS, de Faria GE, Nobre MCL, Machado ACHR, Boggio RF (2023) Rejuvenation of Frontal and Periorbital with Hyaluronan Hybrid Cooperative Complex (HCC): A Novel Technique. J Clin Exp Dermatol Res. 14:653.
Received: 21-Oct-2023, Manuscript No. JCEDR-23-27759; Editor assigned: 23-Oct-2023, Pre QC No. JCEDR-23-27759 (PQ); Reviewed: 06-Nov-2023, QC No. JCEDR-23-27759; Revised: 13-Nov-2023, Manuscript No. JCEDR-23-27759 (R); Published: 20-Nov-2023 , DOI: 10.35841/2329-9509.23.14.653
Copyright: © 2023 Palo JS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.